Difference between revisions of "Glutamate receptor ionotropic, NMDA 2A"
(Created page with "{| align="left" | __TOC__ |} {{#invoke:InfoboxforTarget|run|GluN2A, NMDAR2A|[https://www.uniprot.org/uniprot/Q12879 Q12879]|Homo sapiens|Cys87, Cys320, Cys399|Glutamate-ga...") |
(→Protein Function) |
||
| Line 7: | Line 7: | ||
===Protein Function === | ===Protein Function === | ||
The N-methyl-D-aspartate receptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel protein found in nerve cells. It is activated when glutamate and glycine (or D-serine) bind to it, and when activated it allows positively charged ions to flow through the cell membrane. The NMDA receptor is very important for controlling synaptic plasticity and memory function. <br/> | The N-methyl-D-aspartate receptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel protein found in nerve cells. It is activated when glutamate and glycine (or D-serine) bind to it, and when activated it allows positively charged ions to flow through the cell membrane. The NMDA receptor is very important for controlling synaptic plasticity and memory function. <br/> | ||
| − | The NMDAR is a specific type of ionotropic glutamate receptor. The NMDA receptor is named this because the agonist molecule N-methyl-D-aspartate (NMDA) binds selectively to it, and not to other glutamate receptors. Activation of NMDA receptors results in the opening of an ion channel that is nonselective to cations with a reversal potential near 0 mV. A property of the NMDA receptor is its voltage-dependent activation, a result of ion channel block by extracellular | + | The NMDAR is a specific type of ionotropic glutamate receptor. The NMDA receptor is named this because the agonist molecule N-methyl-D-aspartate (NMDA) binds selectively to it, and not to other glutamate receptors. Activation of NMDA receptors results in the opening of an ion channel that is nonselective to cations with a reversal potential near 0 mV. A property of the NMDA receptor is its voltage-dependent activation, a result of ion channel block by extracellular Mg<sup>2+</sup> & Zn<sup>2+</sup> ions. This allows the flow of Na<sup>+</sup> and small amounts of Ca<sup>2+</sup> ions into the cell and K<sup>+</sup> out of the cell to be voltage-dependent. <br/> |
Calcium flux through NMDARs is thought to be critical in synaptic plasticity, a cellular mechanism for learning and memory. The NMDA receptor is distinct in two ways: first, it is both ligand-gated and voltage-dependent; second, it requires co-activation by two ligands: glutamate and either D-serine or glycine. <br/> | Calcium flux through NMDARs is thought to be critical in synaptic plasticity, a cellular mechanism for learning and memory. The NMDA receptor is distinct in two ways: first, it is both ligand-gated and voltage-dependent; second, it requires co-activation by two ligands: glutamate and either D-serine or glycine. <br/> | ||
The activity of the NMDA receptor is affected by many psychoactive drugs such as phencyclidine (PCP), alcohol (ethanol) and dextromethorphan (DXM). The anaesthetic effects of the drugs ketamine and nitrous oxide are partially because of their effects on NMDA receptor activity. (From Wikipedia)<br/> | The activity of the NMDA receptor is affected by many psychoactive drugs such as phencyclidine (PCP), alcohol (ethanol) and dextromethorphan (DXM). The anaesthetic effects of the drugs ketamine and nitrous oxide are partially because of their effects on NMDA receptor activity. (From Wikipedia)<br/> | ||
Revision as of 03:10, 10 August 2019
| Basic Information | |
|---|---|
| Short Name | GluN2A, NMDAR2A |
| UNP ID | Q12879 |
| Organism | Homo sapiens |
| Cys Site | Cys87, Cys320, Cys399 |
| Family/Domain |
Glutamate-gated ion channel family, NR2A/GRIN2A subfamily |
| Known Ligand | Ligand list |
| Function Type | Ion channel |
Summary
Protein Function
The N-methyl-D-aspartate receptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel protein found in nerve cells. It is activated when glutamate and glycine (or D-serine) bind to it, and when activated it allows positively charged ions to flow through the cell membrane. The NMDA receptor is very important for controlling synaptic plasticity and memory function.
The NMDAR is a specific type of ionotropic glutamate receptor. The NMDA receptor is named this because the agonist molecule N-methyl-D-aspartate (NMDA) binds selectively to it, and not to other glutamate receptors. Activation of NMDA receptors results in the opening of an ion channel that is nonselective to cations with a reversal potential near 0 mV. A property of the NMDA receptor is its voltage-dependent activation, a result of ion channel block by extracellular Mg2+ & Zn2+ ions. This allows the flow of Na+ and small amounts of Ca2+ ions into the cell and K+ out of the cell to be voltage-dependent.
Calcium flux through NMDARs is thought to be critical in synaptic plasticity, a cellular mechanism for learning and memory. The NMDA receptor is distinct in two ways: first, it is both ligand-gated and voltage-dependent; second, it requires co-activation by two ligands: glutamate and either D-serine or glycine.
The activity of the NMDA receptor is affected by many psychoactive drugs such as phencyclidine (PCP), alcohol (ethanol) and dextromethorphan (DXM). The anaesthetic effects of the drugs ketamine and nitrous oxide are partially because of their effects on NMDA receptor activity. (From Wikipedia)
NMDA receptor subtype of glutamate-gated ion channels possesses high calcium permeability and voltage-dependent sensitivity to magnesium. Activation requires binding of agonist to both types of subunits. (From Uniprot)
Cys Function & Property
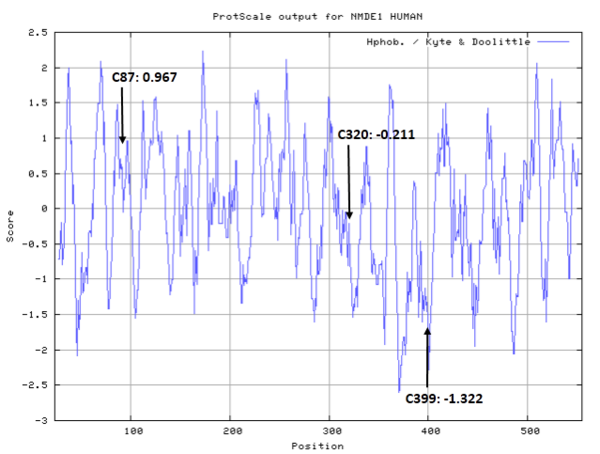
- Hydrophobic property:
- SASA:
- Cys87: Unknown
- Cys320: Unknown
- Cys399: Unknown
Protein Sequence
MGRVGYWTLL VLPALLVWRG PAPSAAAEKG PPALNIAVML GHSHDVTERE
LRTLWGPEQA AGLPLDVNVV ALLMNRTDPK SLITHVCDLM SGARIHGLVF
GDDTDQEAVA QMLDFISSHT FVPILGIHGG ASMIMADKDP TSTFFQFGAS
IQQQATVMLK IMQDYDWHVF SLVTTIFPGY REFISFVKTT VDNSFVGWDM
QNVITLDTSF EDAKTQVQLK KIHSSVILLY CSKDEAVLIL SEARSLGLTG
YDFFWIVPSL VSGNTELIPK EFPSGLISVS YDDWDYSLEA RVRDGIGILT
TAASSMLEKF SYIPEAKASC YGQMERPEVP MHTLHPFMVN VTWDGKDLSF
TEEGYQVHPR LVVIVLNKDR EWEKVGKWEN HTLSLRHAVW PRYKSFSDCE
PDDNHLSIVT LEEAPFVIVE DIDPLTETCV RNTVPCRKFV KINNSTNEGM
NVKKCCKGFC IDILKKLSRT VKFTYDLYLV TNGKHGKKVN NVWNGMIGEV
VYQRAVMAVG SLTINEERSE VVDFSVPFVE TGISVMVSRS NGTVSPSAFL
EPFSASVWVM MFVMLLIVSA IAVFVFEYFS PVGYNRNLAK GKAPHGPSFT
IGKAIWLLWG LVFNNSVPVQ NPKGTTSKIM VSVWAFFAVI FLASYTANLA
AFMIQEEFVD QVTGLSDKKF QRPHDYSPPF RFGTVPNGST ERNIRNNYPY
MHQYMTKFNQ KGVEDALVSL KTGKLDAFIY DAAVLNYKAG RDEGCKLVTI
GSGYIFATTG YGIALQKGSP WKRQIDLALL QFVGDGEMEE LETLWLTGIC
HNEKNEVMSS QLDIDNMAGV FYMLAAAMAL SLITFIWEHL FYWKLRFCFT
GVCSDRPGLL FSISRGIYSC IHGVHIEEKK KSPDFNLTGS QSNMLKLLRS
AKNISSMSNM NSSRMDSPKR AADFIQRGSL IMDMVSDKGN LMYSDNRSFQ
GKESIFGDNM NELQTFVANR QKDNLNNYVF QGQHPLTLNE SNPNTVEVAV
STESKANSRP RQLWKKSVDS IRQDSLSQNP VSQRDEATAE NRTHSLKSPR
YLPEEMAHSD ISETSNRATC HREPDNSKNH KTKDNFKRSV ASKYPKDCSE
VERTYLKTKS SSPRDKIYTI DGEKEPGFHL DPPQFVENVT LPENVDFPDP
YQDPSENFRK GDSTLPMNRN PLHNEEGLSN NDQYKLYSKH FTLKDKGSPH
SETSERYRQN STHCRSCLSN MPTYSGHFTM RSPFKCDACL RMGNLYDIDE
DQMLQETGNP ATGEQVYQQD WAQNNALQLQ KNKLRISRQH SYDNIVDKPR
ELDLSRPSRS ISLKDRERLL EGNFYGSLFS VPSSKLSGKK SSLFPQGLED
SKRSKSLLPD HTSDNPFLHS HRDDQRLVIG RCPSDPYKHS LPSQAVNDSY
LRSSLRSTAS YCSRDSRGHN DVYISEHVMP YAANKNNMYS TPRVLNSCSN
RRVYKKMPSI ESDV
Structural Information
- Known structure with covalent ligand:
- Unknown
- Protein structure:
- Unknown
Related Pathway
- Ras signaling pathway
- Rap1 signaling pathway
- Calcium signaling pathway
- cAMP signaling pathway
- Neuroactive ligand-receptor interaction
- Circadian entrainment
- Long-term potentiation
- Glutamatergic synapse
- Alzheimer disease
- Dopaminergic synapse
- Amyotrophic lateral sclerosis (ALS)
- Cocaine addiction
- Amphetamine addiction
- Nicotine addiction
- Alcoholism
- Systemic lupus erythematosus
Experimental Evidence
- Cys-directed Mutation
Reference
- Kim W K, Choi Y B, Rayudu P V, et al. Attenuation of NMDA receptor activity and neurotoxicity by nitroxyl anion, NO−[J]. Neuron, 1999, 24(2): 461-469. 10571239
- Targets
- Homo sapiens
- Ion channel
- Glutamate-gated ion channel family
- NR1/GRIN1 subfamily
- Ras signaling pathway
- Rap1 signaling pathway
- Calcium signaling pathway
- CAMP signaling pathway
- Neuroactive ligand-receptor interaction
- Circadian entrainment
- Long-term potentiation
- Glutamatergic synapse
- Alzheimer disease
- Amyotrophic lateral sclerosis (ALS)
- Dopaminergic synapse
- Cocaine addiction
- Amphetamine addiction
- Nicotine addiction
- Alcoholism
- Systemic lupus erythematosus