Difference between revisions of "Inosine-5'-monophosphate dehydrogenase 2"
(→Reference) |
(→Reference) |
||
| Line 50: | Line 50: | ||
# Colby T D, Vanderveen K, Strickler M D, et al. '''Crystal structure of human type II inosine monophosphate dehydrogenase: implications for ligand binding and drug design[J].''' Proceedings of the National Academy of Sciences, 1999, 96(7): 3531-3536. [https://www.ncbi.nlm.nih.gov/pubmed/?term=10097070 10097070]<br/> | # Colby T D, Vanderveen K, Strickler M D, et al. '''Crystal structure of human type II inosine monophosphate dehydrogenase: implications for ligand binding and drug design[J].''' Proceedings of the National Academy of Sciences, 1999, 96(7): 3531-3536. [https://www.ncbi.nlm.nih.gov/pubmed/?term=10097070 10097070]<br/> | ||
| − | [[Category: | + | [[Category:Targets]] |
| − | [[Category: | + | [[Category:Homo sapiens]] |
| − | [[Category: | + | [[Category:Metabolic enzyme]] |
| − | [[Category: | + | [[Category:IMPDH/GMPR family]] |
| − | [[Category: | + | [[Category:Purine metabolism]] |
| − | [[Category: | + | [[Category:Drug metabolism - other enzymes]] |
| − | [[Category: | + | [[Category:Metabolic pathways]] |
Latest revision as of 23:02, 19 August 2019
| Basic Information | |
|---|---|
| Short Name | IMPDH2 |
| UNP ID | P12268 |
| Organism | Homo sapiens |
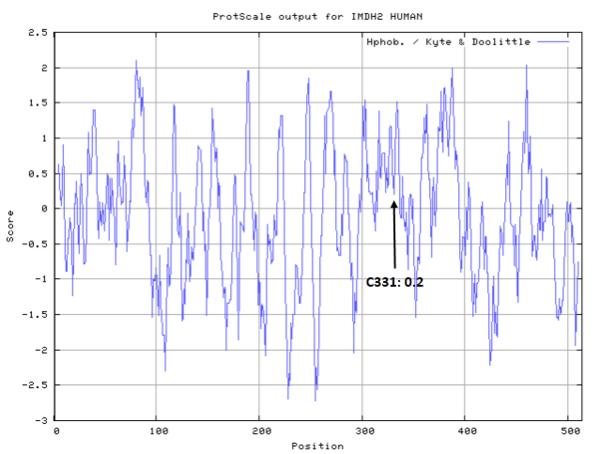
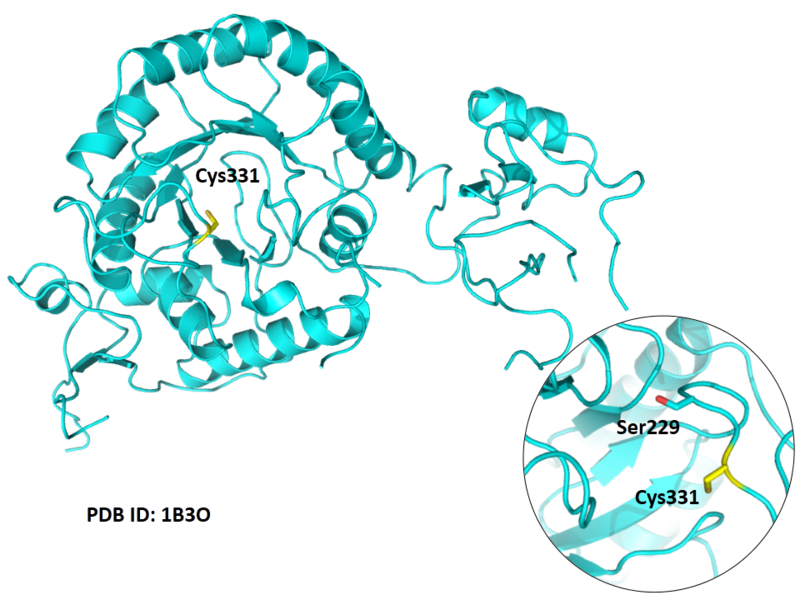
| Cys Site | Cys331 |
| Family/Domain | IMPDH/GMPR family |
| Known Ligand | Ligand list |
| Function Type | Metabolic enzyme |
Summary
Protein Function
IMPDH2 catalyzes the conversion of inosine 5'-phosphate (IMP) to xanthosine 5'-phosphate (XMP), the first committed and rate-limiting step in the de novo synthesis of guanine nucleotides, and therefore plays an important role in the regulation of cell growth. It could also have a single-stranded nucleic acid-binding activity and could play a role in RNA and/or DNA metabolism. It may also have a role in the development of malignancy and the growth progression of some tumors. (From Uniprot)
Cys Function & Property
Cys331 is one of the active sites of IMPDH2, acts as the thioimidate intermediate during the catalytic process.
- Hydrophobic property:
- SASA:
- Cys331: 60.209 A^2
Protein Sequence
MADYLISGGT SYVPDDGLTA QQLFNCGDGL TYNDFLILPG YIDFTADQVD
LTSALTKKIT LKTPLVSSPM DTVTEAGMAI AMALTGGIGF IHHNCTPEFQ
ANEVRKVKKY EQGFITDPVV LSPKDRVRDV FEAKARHGFC GIPITDTGRM
GSRLVGIISS RDIDFLKEEE HDCFLEEIMT KREDLVVAPA GITLKEANEI
LQRSKKGKLP IVNEDDELVA IIARTDLKKN RDYPLASKDA KKQLLCGAAI
GTHEDDKYRL DLLAQAGVDV VVLDSSQGNS IFQINMIKYI KDKYPNLQVI
GGNVVTAAQA KNLIDAGVDA LRVGMGSGSI CITQEVLACG RPQATAVYKV
SEYARRFGVP VIADGGIQNV GHIAKALALG ASTVMMGSLL AATTEAPGEY
FFSDGIRLKK YRGMGSLDAM DKHLSSQNRY FSEADKIKVA QGVSGAVQDK
GSIHKFVPYL IAGIQHSCQD IGAKSLTQVR AMMYSGELKF EKRTSSAQVE
GGVHSLHSYE KRLF
Structural Information
- Known structures with covalent ligands:
- Protein structure:
Related Pathway
Experimental Evidence
- Crystallography, MALDI-TOF/MS, Tryptic Digest, Bio-Rad Protein Assay
Reference
- Wang W, Papov V V, Minakawa N, et al. Inactivation of inosine 5'-monophosphate dehydrogenase by the antiviral agent 5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide 5'-monophosphate[J]. Biochemistry, 1996, 35(1): 95-101. 8555204
- Colby T D, Vanderveen K, Strickler M D, et al. Crystal structure of human type II inosine monophosphate dehydrogenase: implications for ligand binding and drug design[J]. Proceedings of the National Academy of Sciences, 1999, 96(7): 3531-3536. 10097070