Difference between revisions of "Caspase-5"
(→Cys Function & Property) |
(→Protein Function) |
||
| Line 7: | Line 7: | ||
===Protein Function === | ===Protein Function === | ||
Caspase-5 is a member of the cysteine-aspartic acid protease (caspase) family. It is an enzyme that proteolytically cleaves other proteins at an aspartic acid residue y (preferred cleavage after Trp-GluXaa-Asp).<br/> | Caspase-5 is a member of the cysteine-aspartic acid protease (caspase) family. It is an enzyme that proteolytically cleaves other proteins at an aspartic acid residue y (preferred cleavage after Trp-GluXaa-Asp).<br/> | ||
| − | It is an inflammatory caspase, along with caspase-1, caspase-4 and the murine caspase-4 homolog caspase-11, and has a role in the immune system. (From Wikipedia, PMID: 22014003) <br/> | + | |
| + | It is an inflammatory caspase, along with caspase-1, caspase-4 and the murine caspase-4 homolog caspase-11, and has a role in the immune system. (From Wikipedia, PMID: 22014003) <br/> | ||
===Cys Function & Property=== | ===Cys Function & Property=== | ||
Latest revision as of 05:42, 4 January 2020
| Basic Information | |
|---|---|
| Short Name | CASP5 |
| UNP ID | P51878 |
| Organism | Homo sapiens |
| Cys Site | Cys315 |
| Family/Domain | Peptidase C14A family |
| Known Ligand | Ligand list |
| Function Type | Protease |
Summary
Protein Function
Caspase-5 is a member of the cysteine-aspartic acid protease (caspase) family. It is an enzyme that proteolytically cleaves other proteins at an aspartic acid residue y (preferred cleavage after Trp-GluXaa-Asp).
It is an inflammatory caspase, along with caspase-1, caspase-4 and the murine caspase-4 homolog caspase-11, and has a role in the immune system. (From Wikipedia, PMID: 22014003)
Cys Function & Property
The catalytic triad in Caspase-5 comprises Cys315, His267 and Arg221 by similarity.
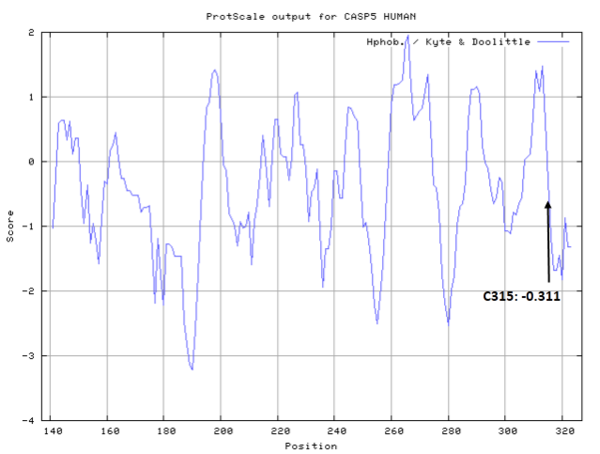
- Hydrophobic property:
- SASA:
- Cys315: Unknown
Protein Sequence
MAEDSGKKKR RKNFEAMFKG ILQSGLDNFV INHMLKNNVA GQTSIQTLVP
NTDQKSTSVK KDNHKKKTVK MLEYLGKDVL HGVFNYLAKH DVLTLKEEEK
KKYYDTKIED KALILVDSLR KNRVAHQMFT QTLLNMDQKI TSVKPLLQIE
AGPPESAEST NILKLCPREE FLRLCKKNHD EIYPIKKRED RRRLALIICN
TKFDHLPARN GAHYDIVGMK RLLQGLGYTV VDEKNLTARD MESVLRAFAA
RPEHKSSDST FLVLMSHGIL EGICGTAHKK KKPDVLLYDT IFQIFNNRNC
LSLKDKPKVI IVQACRGEKH GELWVRDSPA SLALISSQSS ENLEADSVCK
IHEEKDFIAF CSSTPHNVSW RDRTRGSIFI TELITCFQKY SCCCHLMEIF
RKVQKSFEVP QAKAQMPTIE RATLTRDFYL FPGN
Structural Information
- Known structure with covalent ligand:
- Unknown
- Protein structure:
- Unknown
Related Pathway
Experimental Evidence
- Similarity, Site-directed mutation assasy
Reference
- Gao J, Wells J A. Identification of specific tethered inhibitors for caspase‐5[J]. Chemical biology & drug design, 2012, 79(2): 209-215. 22014003