Difference between revisions of "Cathepsin B (Bos taurus)"
(→Reference) |
(→Reference) |
||
| Line 51: | Line 51: | ||
[[Category:Targets|Targets]] | [[Category:Targets|Targets]] | ||
| − | [[Category:Peptidase C1 family|Peptidase C1 family | + | [[Category:Peptidase C1 family|Peptidase C1 family]] |
| − | [[Category:Lysosome|Lysosome | + | [[Category:Lysosome|Lysosome]] |
| − | [[Category:Antigen processing and presentation|Antigen processing and presentation | + | [[Category:Antigen processing and presentation|Antigen processing and presentation]] |
Revision as of 02:58, 28 July 2019
Lua error: data must be either of type string or number.
Summary
Protein Function
CTSB is a thiol protease which is believed to participate in intracellular degradation and turnover of proteins. It has also been implicated in tumor invasion and metastasis.
Hydrolysis of proteins with broad specificity for peptide bonds. Preferentially cleaves -Arg-Arg-|-Xaa bonds in small molecule substrates (thus differing from cathepsin L). In addition to being an endopeptidase, shows peptidyl-dipeptidase activity, liberating C-terminal dipeptides.
Cys Function & Property
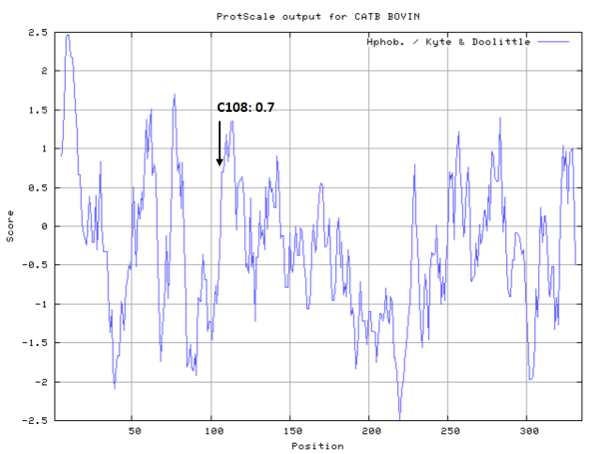
Cys108 is one of the active sites of Cathepsin, which is very close to His278 and Asn298 in spcae. These three residues formed a typical catalytic triad motif.
- Hydrophobic property:
- SASA:
- Cys108: 4.137 A^2
Protein Sequence
MWRLLATLSC LLVLTSARSS LYFPPLSDEL VNFVNKQNTT WKAGHNFYNV
DLSYVKKLCG AILGGPKLPQ RDAFAADVVL PESFDAREQW PNCPTIKEIR
DQGSCGSCWA FGAVEAISDR ICIHSNGRVN VEVSAEDMLT CCGGECGDGC
NGGFPSGAWN FWTKKGLVSG GLYNSHVGCR PYSIPPCEHH VNGSRPPCTG
EGDTPKCSKT CEPGYSPSYK EDKHFGCSSY SVANNEKEIM AEIYKNGPVE
GAFSVYSDFL LYKSGVYQHV SGEIMGGHAI RILGWGVENG TPYWLVGNSW
NTDWGDNGFF KILRGQDHCG IESEIVAGMP CTHQY
Structural Information
- Known structures with covalent ligands:
- Protein structure
Related Pathway
Experimental Evidence
- Crystallography, Nuclear Magnetic Resonance, Homologous Analysis of Sequence
Reference
- Yamamoto A, Tomoo K, Hara T, et al. Substrate specificity of bovine cathepsin B and its inhibition by CA074, based on crystal structure refinement of the complex[J]. The Journal of Biochemistry, 2000, 127(4): 635-643 10739956
- Smith R A, Copp L J, Coles P J, et al. New inhibitors of cysteine proteinases. Peptidyl acyloxymethyl ketones and the quiescent nucleofuge strategy[J]. Journal of the American Chemical Society, 1988, 110(13): 4429-4431. DOI: 10.1021/ja00221a062
- Štern I, Schaschke N, Moroder L, et al. Crystal structure of NS-134 in complex with bovine cathepsin B: a two-headed epoxysuccinyl inhibitor extends along the entire active-site cleft[J]. Biochemical Journal, 2004, 381(2): 511-517.15084146
- Yamamoto A, Tomoo K, Matsugi K, et al. Structural basis for development of cathepsin B-specific noncovalent-type inhibitor: crystal structure of cathepsin B–E64c complex[J]. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology, 2002, 1597(2): 244-251. 12044902
- Watanabe D, Yamamoto A, Tomoo K, et al. Quantitative evaluation of each catalytic subsite of cathepsin B for inhibitory activity based on inhibitory activity–binding mode relationship of epoxysuccinyl inhibitors by X-ray crystal structure analyses of complexes[J]. Journal of molecular biology, 2006, 362(5): 979-993. 16950396