Difference between revisions of "Cathepsin L2"
(→Structural Information) |
|||
| Line 2: | Line 2: | ||
| __TOC__ | | __TOC__ | ||
|} | |} | ||
| − | {{#invoke:InfoboxforTarget|run|CTSV|[https://www.uniprot.org/uniprot/O60911 O60911]|Homo sapiens| | + | {{#invoke:InfoboxforTarget|run|CTSV|[https://www.uniprot.org/uniprot/O60911 O60911]|Homo sapiens|Cys138|[http://pfam.xfam.org/family/PF00112 Peptidase C1 family]|[[:Category:Cathepsin L2|Ligand list]]}} |
==Summary== | ==Summary== | ||
Revision as of 17:49, 28 July 2019
Lua error: data must be either of type string or number.
Summary
Protein Function
Cathepsin L2, also known as cathepsin V and encoded by the CTSL2 gene, is a human gene. The protein encoded by this gene, a member of the peptidase C1 family, is a lysosomal cysteine proteinase that may play an important role in corneal physiology. This gene is expressed in colorectal and breast carcinomas but not in normal colon, mammary gland, or peritumoral tissues, suggesting a possible role for this gene in tumor processes. (From Wikipedia)
Cathepsin V is a lysosomal cysteine protease that is expressed in the thymus, testis and corneal epithelium. Its sequence shows that it is a member of the papain family of cysteine proteases, and several pieces of evidence demonstrate an especially close link between this enzyme and cathepsin L. The sequences of these two enzymes are quite similar, with a sequence identity of 77% for the proenzyme and 80% for the mature enzyme. In addition, the position and chromosomal organization of the genes coding for these two cathepsins are similar. The cathepsin V gene has been mapped to a region near the cathepsin L locus, and both genes consist of eight exons that code for analogous regions of the cathepsins V and L structures. Cathepsin V may be involved in T-cell selection in humans. (PMID: 11027133)
Cys Function & Property
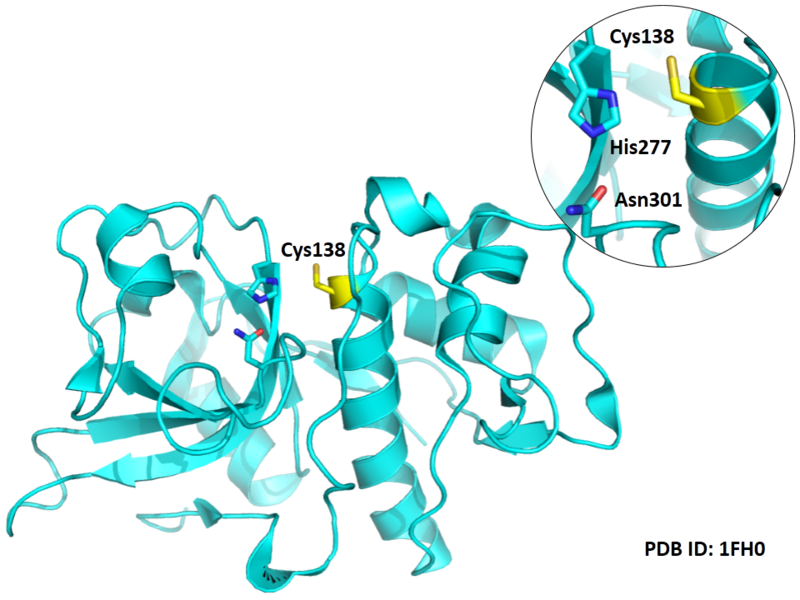
- Cys138 is one of the active sites of Cathepsin L2, which is very close to His277 and Asn301 in space. These three residues formed a typical catalytic triad motif.
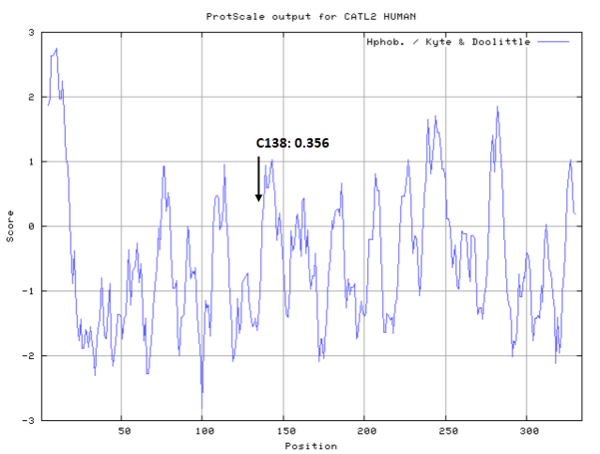
- Hydrophobic property:
- SASA:
- Cys138: 10.412 A^2
Protein Sequence
MNLSLVLAAF CLGIASAVPK FDQNLDTKWY QWKATHRRLY GANEEGWRRA
VWEKNMKMIE LHNGEYSQGK HGFTMAMNAF GDMTNEEFRQ MMGCFRNQKF
RKGKVFREPL FLDLPKSVDW RKKGYVTPVK NQKQCGSCWA FSATGALEGQ
MFRKTGKLVS LSEQNLVDCS RPQGNQGCNG GFMARAFQYV KENGGLDSEE
SYPYVAVDEI CKYRPENSVA NDTGFTVVAP GKEKALMKAV ATVGPISVAM
DAGHSSFQFY KSGIYFEPDC SSKNLDHGVL VVGYGFEGAN SNNSKYWLVK
NSWGPEWGSN GYVKIAKDKN NHCGIATAAS YPNV
Structural Information
- Known structures with covalent ligands:
- Protein structure
Related Pathway
Experimental Evidence
- Crystallography
Reference
- Somoza J R, Zhan H, Bowman K K, et al. Crystal structure of human cathepsin V[J]. Biochemistry, 2000, 39(41): 12543-12551. 11027133