Difference between revisions of "Cathepsin K"
(→Structural Information) |
|||
| Line 2: | Line 2: | ||
| __TOC__ | | __TOC__ | ||
|} | |} | ||
| − | {{#invoke:InfoboxforTarget|run|Cat K, CTSK|[https://www.uniprot.org/uniprot/P43235 P43235]|Homo sapiens|Cys139|[http://pfam.xfam.org/family/PF00112 Peptidase C1 family]|[[:Category:Cathepsin K|Ligand list]]}} | + | {{#invoke:InfoboxforTarget|run|Cat K, CTSK|[https://www.uniprot.org/uniprot/P43235 P43235]|Homo sapiens|Cys139|[http://pfam.xfam.org/family/PF00112 Peptidase C1 family]|[[:Category:Cathepsin K|Ligand list]]|Protease}} |
==Summary== | ==Summary== | ||
| Line 70: | Line 70: | ||
[[Category:Targets|Targets]] | [[Category:Targets|Targets]] | ||
[[Category:Homo sapiens|Homo sapiens]] | [[Category:Homo sapiens|Homo sapiens]] | ||
| + | [[Category:Protease|Protease]] | ||
[[Category:Peptidase C1 family|Peptidase C1 family]] | [[Category:Peptidase C1 family|Peptidase C1 family]] | ||
[[Category:Lysosome|Lysosome]] | [[Category:Lysosome|Lysosome]] | ||
Revision as of 20:35, 29 July 2019
| Basic Information | |
|---|---|
| Short Name | Cat K, CTSK |
| UNP ID | P43235 |
| Organism | Homo sapiens |
| Cys Site | Cys139 |
| Family/Domain | Peptidase C1 family |
| Known Ligand | Ligand list |
| Function Type | Protease |
Summary
Protein Function
Cathepsin K, abbreviated CTSK, is a member of the peptidase C1 protein family, is expressed predominantly in osteoclasts. Cathepsin K could catabolize elastin, collagen, and gelatin allow it to break down bone and cartilage. This catabolic activity is also partially responsible for the loss of lung elasticity and recoil in emphysema. Cathepsin K inhibitors, such as odanacatib, show great potential in the treatment of osteoporosis. Cathepsin K is degraded by Cathepsin S, called Controlled Cathepsin Cannibalism.
Its expression is stimulated by inflammatory cytokines that are released after tissue injury. (From Wikipedia)
Closely involved in osteoclastic bone resorption and may participate partially in the disorder of bone remodeling. Displays potent endoprotease activity against fibrinogen at acid pH. May play an important role in extracellular matrix degradation. (From Uniprot)
Cys Function & Property
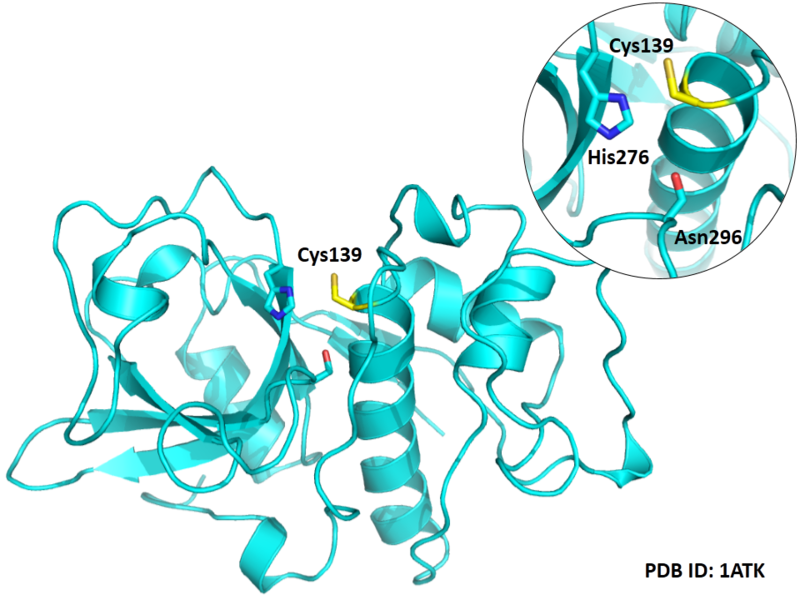
Cys139 is one of the active sites of Cathepsin K, which is very close to His276 and Asn296 in space. These three residues formed a typical catalytic triad motif.
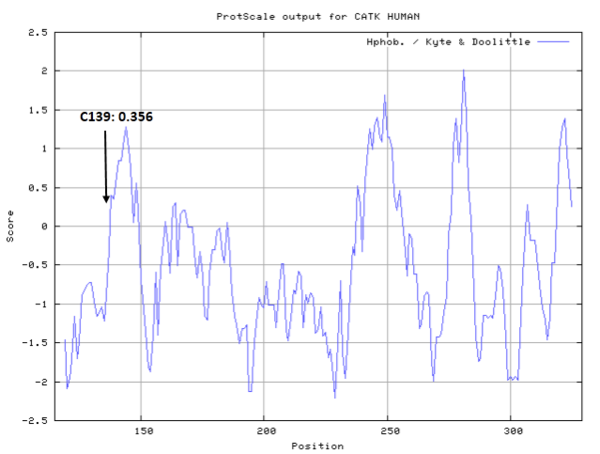
- Hydrophobic property:
- SASA:
- Cys139: 11.382 A^2
Protein Sequence
MWGLKVLLLP VVSFALYPEE ILDTHWELWK KTHRKQYNNK VDEISRRLIW
EKNLKYISIH NLEASLGVHT YELAMNHLGD MTSEEVVQKM TGLKVPLSHS
RSNDTLYIPE WEGRAPDSVD YRKKGYVTPV KNQGQCGSCW AFSSVGALEG
QLKKKTGKLL NLSPQNLVDC VSENDGCGGG YMTNAFQYVQ KNRGIDSEDA
YPYVGQEESC MYNPTGKAAK CRGYREIPEG NEKALKRAVA RVGPVSVAID
ASLTSFQFYS KGVYYDESCN SDNLNHAVLA VGYGIQKGNK HWIIKNSWGE
NWGNKGYILM ARNKNNACGI ANLASFPKM
Structural Information
- Known structures with covalent ligands:
- 3H7D, 3C9E, 1ATK, 1YT7, 1Q6K, 1SNK
- 1U9V, 1U9W, 1U9X, 1VSN, 1YK7, 1NLJ
- 1YK8, 2AUX, 2AUZ, 3KW9, 1AU0, 1AYW
- 1AU2, 1AU3, 1AU4, 1AYU
- Protein structure:
Related Pathway
- Lysosome
- Apoptosis
- Osteoclast differentiation
- Toll-like receptor signaling pathway
- Rheumatoid arthritis
Experimental Evidence
- Crystallography
Reference
- Cherney M M, Lecaille F, Kienitz M, et al. Structure-activity analysis of cathepsin K/chondroitin 4-sulfate interactions[J]. Journal of Biological Chemistry, 2011, 286(11): 8988-8998. 21193413
- Li Z, Kienetz M, Cherney M M, et al. The crystal and molecular structures of a cathepsin K: chondroitin sulfate complex[J]. Journal of molecular biology, 2008, 383(1): 78-91. 18692071
- Zhao B, Janson C A, Amegadzie B Y, et al. Crystal structure of human osteoclast cathepsin K complex with E-64[J]. Nature structural biology, 1997, 4(2): 109. 9033588
- Respondek T, Garner R N, Herroon M K, et al. Light activation of a cysteine protease inhibitor: caging of a peptidomimetic nitrile with RuII(bpy)2[J]. Journal of the American Chemical Society, 2011, 133(43): 17164-17167. 21973207
- Barrett D G, Boncek V M, Catalano J G, et al. P2–P3 conformationally constrained ketoamide-based inhibitors of cathepsin K[J]. Bioorganic & medicinal chemistry letters, 2005, 15(15): 3540-3546. 15982880
- Catalano J G, Deaton D N, Furfine E S, et al. Exploration of the P1 SAR of aldehyde cathepsin K inhibitors[J]. Bioorganic & medicinal chemistry letters, 2004, 14(1): 275-278. 14684342
- Li C S, Deschenes D, Desmarais S, et al. Identification of a potent and selective non-basic cathepsin K inhibitor[J]. Bioorganic & medicinal chemistry letters, 2006, 16(7): 1985-1989. 16413777
- Deaton D N, Hassell A M, McFadyen R B, et al. Novel and potent cyclic cyanamide-based cathepsin K inhibitors[J]. Bioorganic & medicinal chemistry letters, 2005, 15(7): 1815-1819. 15780613
- Barrett D G, Deaton D N, Hassell A M, et al. Acyclic cyanamide-based inhibitors of cathepsin K[J]. Bioorganic & medicinal chemistry letters, 2005, 15(12): 3039-3043. 15896958
- Adkison K K, Barrett D G, Deaton D N, et al. Semicarbazone-based inhibitors of cathepsin K, are they prodrugs for aldehyde inhibitors?[J]. Bioorganic & medicinal chemistry letters, 2006, 16(4): 978-983. 16290936
- Rankovic Z, Cai J, Kerr J, et al. Design and optimization of a series of novel 2-cyano-pyrimidines as cathepsin K inhibitors[J]. Bioorganic & medicinal chemistry letters, 2010, 20(5): 1524-1527. 20149657
- DesJarlais R L, Yamashita D S, Oh H J, et al. Use of X-ray co-crystal structures and molecular modeling to design potent and selective non-peptide inhibitors of cathepsin K[J]. Journal of the American Chemical Society, 1998, 120(35): 9114-9115. DOI:10.1021/ja981171v
- Marquis R W, Yamashita D S, Ru Y, et al. Conformationally constrained 1, 3-diamino ketones: a series of potent inhibitors of the cysteine protease cathepsin K[J]. Journal of medicinal chemistry, 1998, 41(19): 3563-3567. 9733481
- Thompson S K, Halbert S M, Bossard M J, et al. Design of potent and selective human cathepsin K inhibitors that span the active site[J]. Proceedings of the National Academy of Sciences, 1997, 94(26): 14249-14254. 9405598
- Marquis R W, Ru Y, LoCastro S M, et al. Azepanone-based inhibitors of human and rat cathepsin K[J]. Journal of medicinal chemistry, 2001, 44(9): 1380-1395. 11311061
- Boros E E, Deaton D N, Hassell A M, et al. Exploration of the P2–P3 SAR of aldehyde cathepsin K inhibitors[J]. Bioorganic & medicinal chemistry letters, 2004, 14(13): 3425-3429. 15177446