Difference between revisions of "GTPase HRas"
(→Cys Function & Property) |
(→Structural Information) |
||
| Line 32: | Line 32: | ||
*Protein structure: | *Protein structure: | ||
| − | ::[[File:508.png||800px]] | + | ::[[File:508.png|center|800px]] |
==Related Pathway== | ==Related Pathway== | ||
Revision as of 03:56, 31 July 2019
| Basic Information | |
|---|---|
| Short Name | HRAS, p21ras |
| UNP ID | P01112 |
| Organism | Homo sapiens |
| Cys Site | Cys118, Cys184 |
| Family/Domain |
Ras family, Small GTPase superfamily |
| Known Ligand | Ligand list |
| Function Type | ATPase/GTPase |
Summary
Protein Function
Ras proteins bind GDP/GTP and possess intrinsic GTPase activity. (From Uniprot)
GTPase HRas also known as transforming protein p21 is an enzyme that in humans is encoded by the HRAS gene. The HRAS gene is located on the short (p) arm of chromosome 11 at position 15.5, from base pair 522,241 to base pair 525,549. HRas is a small G protein in the Ras subfamily of the Ras superfamily of small GTPases. Once bound to Guanosine triphosphate, H-Ras will activate a Raf kinase like c-Raf, the next step in the MAPK/ERK pathway. GTPase HRas is involved in regulating cell division in response to growth factor stimulation. Growth factors act by binding cell surface receptors that span the cell's plasma membrane. Once activated, receptors stimulate signal transduction events in the cytoplasm, a process by which proteins and second messengers relay signals from outside the cell to the cell nucleus and instructs the cell to grow or divide. The HRAS protein is a GTPase and is an early player in many signal transduction pathways and is usually associated with cell membranes due to the presence of an isoprenyl group on its C-terminus. HRAS acts as a molecular on/off switch, once it is turned on it recruits and activates proteins necessary for the propagation of the receptor's signal, such as c-Raf and PI 3-kinase. HRAS binds to GTP in the active state and possesses an intrinsic enzymatic activity that cleaves the terminal phosphate of this nucleotide converting it to GDP. Upon conversion of GTP to GDP, HRAS is turned off. The rate of conversion is usually slow but can be sped up dramatically by an accessory protein of the GTPase activating protein (GAP) class, for example RasGAP. In turn HRAS can bind to proteins of the Guanine Nucleotide Exchange Factor (GEF) class, for example SOS1, which forces the release of bound nucleotide. Subsequently, GTP present in the cytosol binds and HRAS-GTP dissociates from the GEF, resulting in HRAS activation. HRAS is in the Ras family, which also includes two other proto-oncogenes: KRAS and NRAS. These proteins all are regulated in the same manner and appear to differ largely in their sites of action within the cell. (From Wiki)
Cys Function & Property
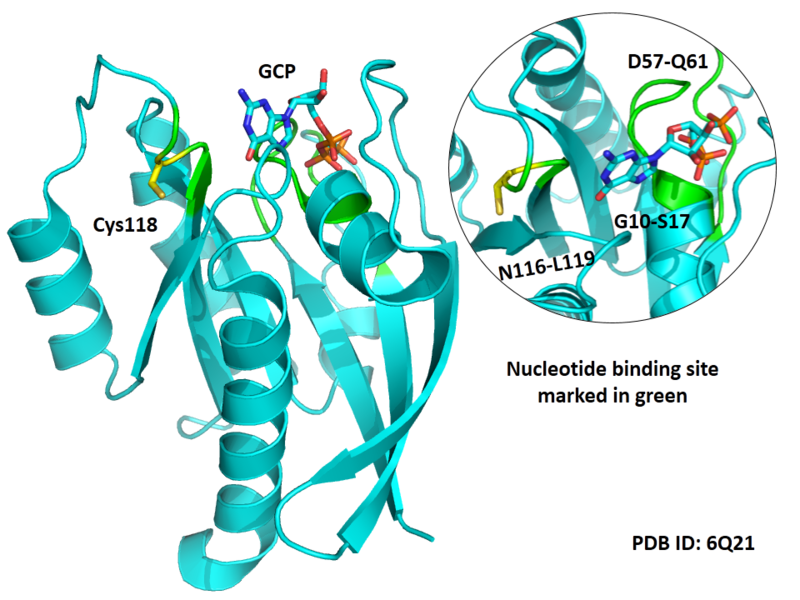
Cys118 is used to bind with GTP in HRas.
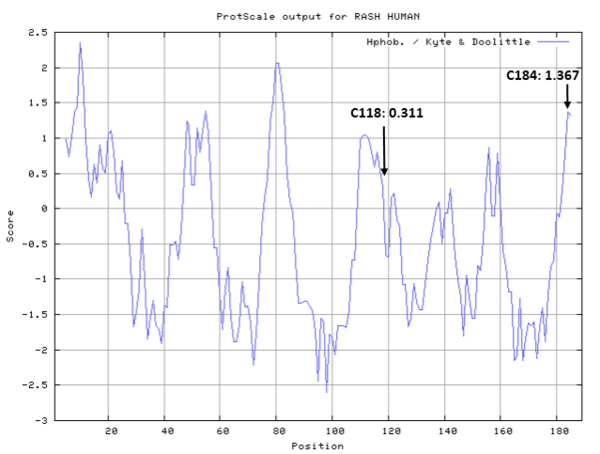
- Hydrophobic property:
- SASA:
- Cys118: 270148 A^2
- Cys184: Unknown
Protein Sequence
MTEYKLVVVG AGGVGKSALT IQLIQNHFVD EYDPTIEDSY RKQVVIDGET
CLLDILDTAG QEEYSAMRDQ YMRTGEGFLC VFAINNTKSF EDIHQYREQI
KRVKDSDDVP MVLVGNKCDL AARTVESRQA QDLARSYGIP YIETSAKTRQ
GVEDAFYTLV REIRQHKLRK LNPPDESGPG CMSCKCVLS
Structural Information
- Known structure with covalent ligand:
- Protein structure:
Related Pathway
- EGFR tyrosine kinase inhibitor resistance
- Endocrine resistance
- MAPK signaling pathway
- ErbB signaling pathway
- Ras signaling pathway
- Rap1 signaling pathway
- Chemokine signaling pathway
- FoxO signaling pathway
- Sphingolipid signaling pathway
- Phospholipase D signaling pathway
- Mitophagy - animal
- Autophagy - animal
- Endocytosis
- mTOR signaling pathway
- PI3K-Akt signaling pathway
- Apoptosis
- Longevity regulating pathway
- Cellular senescence
- Axon guidance
- VEGF signaling pathway
- Apelin signaling pathway
- Gap junction
- Signaling pathways regulating pluripotency of stem cells
- C-type lectin receptor signaling pathway
- Jak-STAT signaling pathway
- Natural killer cell mediated cytotoxicity
- T cell receptor signaling pathway
- B cell receptor signaling pathway
- Fc epsilon RI signaling pathway
- Thermogenesis
- Long-term potentiation
- Neurotrophin signaling pathway
- Cholinergic synapse
- Serotonergic synapse
- Long-term depression
- Regulation of actin cytoskeleton
- Insulin signaling pathway
- GnRH signaling pathway
- Estrogen signaling pathway
- Melanogenesis
- Prolactin signaling pathway
- Thyroid hormone signaling pathway
- Oxytocin signaling pathway
- Relaxin signaling pathway
- AGE-RAGE signaling pathway in diabetic complications
- Alcoholism
- Hepatitis C
- Hepatitis B
- Human cytomegalovirus infection
- Human papillomavirus infection
- Human T-cell leukemia virus 1 infection
- Kaposi sarcoma-associated herpesvirus infection
- Human immunodeficiency virus 1 infection
- Pathways in cancer
- Viral carcinogenesis
- Proteoglycans in cancer
- MicroRNAs in cancer
- Colorectal cancer
- Renal cell carcinoma
- Endometrial cancer
- Glioma
- Prostate cancer
- Thyroid cancer
- Melanoma
- Bladder cancer
- Chronic myeloid leukemia
- Acute myeloid leukemia
- Non-small cell lung cancer
- Breast cancer
- Hepatocellular carcinoma
- Gastric cancer
- Central carbon metabolism in cancer
- Choline metabolism in cancer
- PD-L1 expression and PD-1 checkpoint pathway in cancer
Experimental Evidence
- Crystallography, LC-ESI-MS/MS, Tryptic Digest, Cys-directed Mutation, Western Blot
Reference
- Oliva J L, Pérez-Sala D, Castrillo A, et al. The cyclopentenone 15-deoxy-Δ12,14-prostaglandin J2 binds to and activates H-Ras[J]. Proceedings of the National Academy of Sciences, 2003, 100(8): 4772-4777. 12684535
- Springstead J R, Gugiu B G, Lee S, et al. Evidence for the importance of OxPAPC interaction with cysteines in regulating endothelial cell function[J]. Journal of lipid research, 2012, 53(7): 1304-1315. 22550136
- Sredni B, Geffen-Aricha R, Duan W, et al. Multifunctional tellurium molecule protects and restores dopaminergic neurons in Parkinson’s disease models[J]. The FASEB Journal, 2007, 21(8): 1870-1883. 17314138
- Winter J J G, Anderson M, Blades K, et al. Small molecule binding sites on the Ras: SOS complex can be exploited for inhibition of Ras activation[J]. Journal of medicinal chemistry, 2015, 58(5): 2265-2274. 25695162
- Targets
- Homo sapiens
- ATPase/GTPase
- Small GTPase superfamily
- Ras family
- EGFR tyrosine kinase inhibitor resistance
- Endocrine resistance
- MAPK signaling pathway
- ErbB signaling pathway
- Ras signaling pathway
- Rap1 signaling pathway
- Chemokine signaling pathway
- FoxO signaling pathway
- Sphingolipid signaling pathway
- Mitophagy - animal
- Autophagy - animal
- Endocytosis
- MTOR signaling pathway
- PI3K-Akt signaling pathway
- Apoptosis
- Longevity regulating pathway
- Cellular senescence
- Axon guidance
- VEGF signaling pathway
- Apelin signaling pathway
- Gap junction
- Signaling pathways regulating pluripotency of stem cells
- C-type lectin receptor signaling pathway
- Jak-STAT signaling pathway
- Natural killer cell mediated cytotoxicity
- T cell receptor signaling pathway
- B cell receptor signaling pathway
- Fc epsilon RI signaling pathway
- Thermogenesis
- Long-term potentiation
- Neurotrophin signaling pathway
- Cholinergic synapse
- Serotonergic synapse
- Long-term depression
- Regulation of actin cytoskeleton
- Insulin signaling pathway
- GnRH signaling pathway
- Estrogen signaling pathway
- Melanogenesis
- Prolactin signaling pathway
- Thyroid hormone signaling pathway
- Oxytocin signaling pathway
- Relaxin signaling pathway
- AGE-RAGE signaling pathway in diabetic complications
- Alcoholism
- Hepatitis C
- Hepatitis B
- Human cytomegalovirus infection
- Human papillomavirus infection
- Human T-cell leukemia virus 1 infection
- Kaposi sarcoma-associated herpesvirus infection
- Human immunodeficiency virus 1 infection
- Pathways in cancer
- Viral carcinogenesis
- Proteoglycans in cancer
- MicroRNAs in cancer
- Colorectal cancer
- Renal cell carcinoma
- Endometrial cancer
- Glioma
- Prostate cancer
- Thyroid cancer
- Melanoma
- Bladder cancer
- Chronic myeloid leukemia
- Acute myeloid leukemia
- Non-small cell lung cancer
- Breast cancer
- Hepatocellular carcinoma
- Gastric cancer
- Central carbon metabolism in cancer
- Choline metabolism in cancer
- PD-L1 expression and PD-1 checkpoint pathway in cancer