Difference between revisions of "Cofilin-1"
(Created page with "{| align="left" | __TOC__ |} {{#invoke:InfoboxforTarget|run|Cofilin-1, p18|[https://www.uniprot.org/uniprot/P23528 P23528]|Homo sapiens|Cys139|[http://pfam.xfam.org/family...") |
(→Reference) |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
| __TOC__ | | __TOC__ | ||
|} | |} | ||
| − | {{#invoke:InfoboxforTarget|run|Cofilin-1, p18|[https://www.uniprot.org/uniprot/P23528 P23528]|Homo sapiens|Cys139|[http://pfam.xfam.org/family/PF00241 Cofilin/tropomyosin-type actin-binding protein],<br/>Actin-binding proteins ADF family | + | {{#invoke:InfoboxforTarget|run|Cofilin-1, p18|[https://www.uniprot.org/uniprot/P23528 P23528]|Homo sapiens|Cys139|[http://pfam.xfam.org/family/PF00241 Cofilin/tropomyosin-type actin-binding protein],<br/>Actin-binding proteins ADF family|[[:Category:Cofilin-1|Ligand list]]|Structural protein}} |
==Summary== | ==Summary== | ||
| Line 11: | Line 11: | ||
===Cys Function & Property=== | ===Cys Function & Property=== | ||
| − | + | Molecules covalent bind with Cys139 would disrupt the interface between Cofilin1 and actin.<br/> | |
* Hydrophobic property: | * Hydrophobic property: | ||
| Line 22: | Line 22: | ||
MASGVAVSDG VIKVFNDMKV RKSSTPEEVK KRKKAVLFCL SEDKKNIILE <br/> | MASGVAVSDG VIKVFNDMKV RKSSTPEEVK KRKKAVLFCL SEDKKNIILE <br/> | ||
EGKEILVGDV GQTVDDPYAT FVKMLPDKDC RYALYDATYE TKESKKEDLV <br/> | EGKEILVGDV GQTVDDPYAT FVKMLPDKDC RYALYDATYE TKESKKEDLV <br/> | ||
| − | FIFWAPESAP LKSKMIYASS KDAIKKKLTG | + | FIFWAPESAP LKSKMIYASS KDAIKKKLTG IKHELQAN<span style="background:#ffff00">'''C'''</span>Y EEVKDRCTLA <br/> |
| − | |||
| − | |||
EKLGGSAVIS LEGKPL <br/> | EKLGGSAVIS LEGKPL <br/> | ||
</font> | </font> | ||
| Line 48: | Line 46: | ||
# Gabrielsen M, Schuldt M, Munro J, et al. '''Cucurbitacin covalent bonding to cysteine thiols: the filamentous-actin severing protein Cofilin1 as an exemplary target[J].''' Cell Communication and Signaling, 2013, 11(1): 58. [https://www.ncbi.nlm.nih.gov/pubmed/?term=23945128 23945128]<br/> | # Gabrielsen M, Schuldt M, Munro J, et al. '''Cucurbitacin covalent bonding to cysteine thiols: the filamentous-actin severing protein Cofilin1 as an exemplary target[J].''' Cell Communication and Signaling, 2013, 11(1): 58. [https://www.ncbi.nlm.nih.gov/pubmed/?term=23945128 23945128]<br/> | ||
| − | [[Category: | + | [[Category:Targets]] |
| − | [[Category: | + | [[Category:Homo sapiens]] |
| − | [[Category: | + | [[Category:Structural protein]] |
| − | [[Category: | + | [[Category:Actin-binding proteins ADF family]] |
| − | [[Category: | + | [[Category:Fc gamma R-mediated phagocytosis]] |
| − | [[Category: | + | [[Category:Regulation of actin cytoskeleton]] |
| − | [[Category: | + | [[Category:Pertussis]] |
| − | [[Category: | + | [[Category:Human immunodeficiency virus 1 infection]] |
Latest revision as of 22:06, 19 August 2019
| Basic Information | |
|---|---|
| Short Name | Cofilin-1, p18 |
| UNP ID | P23528 |
| Organism | Homo sapiens |
| Cys Site | Cys139 |
| Family/Domain |
Cofilin/tropomyosin-type actin-binding protein, Actin-binding proteins ADF family |
| Known Ligand | Ligand list |
| Function Type | Structural protein |
Summary
Protein Function
Cofilin is a widely distributed intracellular actin-modulating protein that binds and depolymerizes filamentous F-actin and inhibits the polymerization of monomeric G-actin in a pH-dependent manner. It is involved in the translocation of actin-cofilin complex from cytoplasm to nucleus.
One group reports that reelin signaling leads to serine3-phosphorylation of cofilin-1, and this interaction may play a role in the reelin-related regulation of neuronal migration.
Cys Function & Property
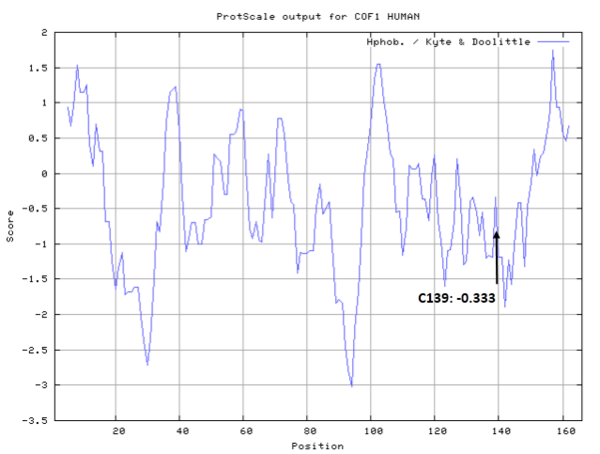
Molecules covalent bind with Cys139 would disrupt the interface between Cofilin1 and actin.
- Hydrophobic property:
- SASA:
- Cys139: 36.275 A^2
Protein Sequence
MASGVAVSDG VIKVFNDMKV RKSSTPEEVK KRKKAVLFCL SEDKKNIILE
EGKEILVGDV GQTVDDPYAT FVKMLPDKDC RYALYDATYE TKESKKEDLV
FIFWAPESAP LKSKMIYASS KDAIKKKLTG IKHELQANCY EEVKDRCTLA
EKLGGSAVIS LEGKPL
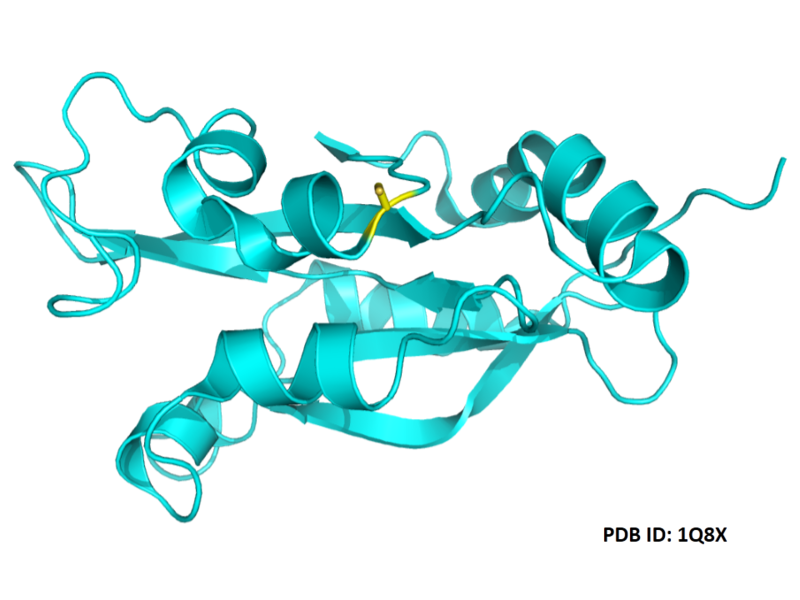
Structural Information
- Known structures with covalent ligands:
- Unknown
- Protein structure:
Related Pathway
- Axon guidance
- Fc gamma R-mediated phagocytosis
- Regulation of actin cytoskeleton
- Pertussis
- Human immunodeficiency virus 1 infection
Experimental Evidence
- Mass Spectrometry, Tryptic Digest
Reference
- Gabrielsen M, Schuldt M, Munro J, et al. Cucurbitacin covalent bonding to cysteine thiols: the filamentous-actin severing protein Cofilin1 as an exemplary target[J]. Cell Communication and Signaling, 2013, 11(1): 58. 23945128