Difference between revisions of "Calpain-1"
(→Protein Function) |
(→Reference) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 2: | Line 2: | ||
| __TOC__ | | __TOC__ | ||
|} | |} | ||
| − | {{#invoke:InfoboxforTarget|run|CAPN1|[https://www.uniprot.org/uniprot/P97571 P97571]|Rattus norvegicus|Cys115|[http://pfam.xfam.org/family/PF00648 Peptidase C2 family]|[[:Category:Calpain-1|Ligand list]]}} | + | {{#invoke:InfoboxforTarget|run|CAPN1|[https://www.uniprot.org/uniprot/P97571 P97571]|Rattus norvegicus|Cys115|[http://pfam.xfam.org/family/PF00648 Peptidase C2 family]|[[:Category:Calpain-1|Ligand list]]|Protease}} |
==Summary== | ==Summary== | ||
| Line 57: | Line 57: | ||
# Moldoveanu T, Campbell R L, Cuerrier D, et al. '''Crystal structures of calpain–E64 and–leupeptin inhibitor complexes reveal mobile loops gating the active site[J].''' Journal of molecular biology, 2004, 343(5): 1313-1326. [https://www.ncbi.nlm.nih.gov/pubmed/?term=15491615 15491615]<br/> | # Moldoveanu T, Campbell R L, Cuerrier D, et al. '''Crystal structures of calpain–E64 and–leupeptin inhibitor complexes reveal mobile loops gating the active site[J].''' Journal of molecular biology, 2004, 343(5): 1313-1326. [https://www.ncbi.nlm.nih.gov/pubmed/?term=15491615 15491615]<br/> | ||
| − | [[Category: | + | [[Category:Targets]] |
| − | [[Category:Rattus norvegicus | + | [[Category:Rattus norvegicus]] |
| − | [[Category: | + | [[Category:Protease]] |
| − | [[Category: | + | [[Category:Peptidase C2 family]] |
| − | [[Category: | + | [[Category:Protein processing in endoplasmic reticulum]] |
| − | [[Category: | + | [[Category:Apoptosis]] |
| − | [[Category: | + | [[Category:Necroptosis]] |
| − | [[Category: | + | [[Category:Cellular senescence]] |
| + | [[Category:Alzheimer disease]] | ||
Latest revision as of 21:38, 19 August 2019
| Basic Information | |
|---|---|
| Short Name | CAPN1 |
| UNP ID | P97571 |
| Organism | Rattus norvegicus |
| Cys Site | Cys115 |
| Family/Domain | Peptidase C2 family |
| Known Ligand | Ligand list |
| Function Type | Protease |
Summary
Protein Function
Calpain-1 catalytic subunit, encoded by the CAPN1 gene, is one type of non-lysosomal, intracellular cysteine proteases. And Calpain-1 is calcium-activated neutral protease. The mammalian calpains include ubiquitous, stomach-specific, and muscle-specific proteins. The ubiquitous enzymes consist of heterodimers with distinct large, catalytic subunits associated with a common small, regulatory subunit. (From Wikipedia)
Calcium-regulated non-lysosomal thiol-protease which catalyze limited proteolysis of substrates involved in cytoskeletal remodeling and signal transduction. Broad endopeptidase specificity. (From Uniprot)
Cys Function & Property
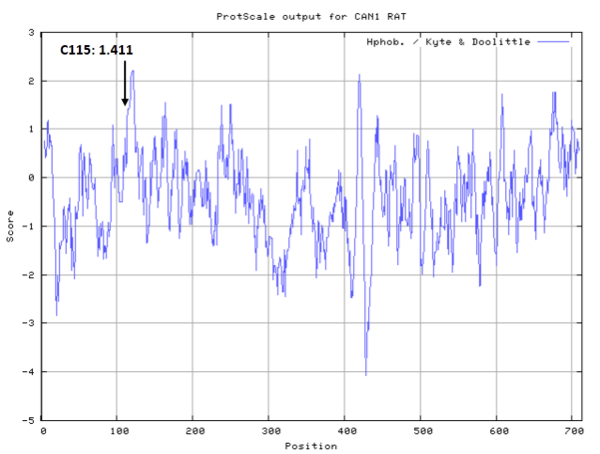
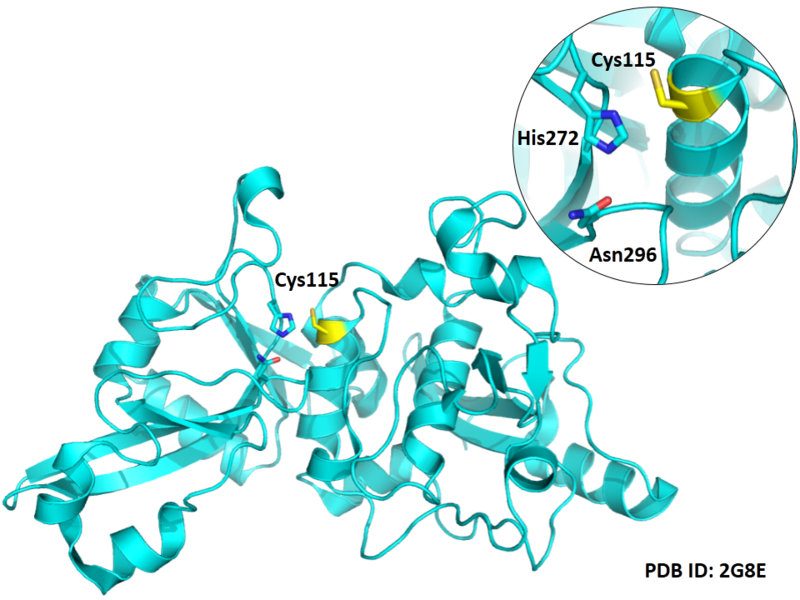
Cys115 is one of the active sites of Cathepsin H, which is very close to His272 and Asn296 in space. These three residues formed a typical catalytic triad motif.
- Hydrophobic property:
- SASA:
- Cys115: 12.816 A^2
Protein Sequence
MAEELITPVY CTGVSAQVQK QRDKELGLGR HENAIKYLGQ DYENLRARCL
QNGVLFQDDA FPPVSHSLGF KELGPNSSKT YGIKWKRPTE LLSNPQFIVD
GATRTDICQG ALGDCWLLAA IASLTLNETI LHRVVPYGQS FQEGYAGIFH
FQLWQFGEWV DVVVDDLLPT KDGKLVFVHS AQGNEFWSAL LEKAYAKVNG
SYEALSGGCT SEAFEDFTGG VTEWYDLQKA PSDLYQIILK ALERGSLLGC
SINISDIRDL EAITFKNLVR GHAYSVTDAK QVTYQGQRVN LIRMRNPWGE
VEWKGPWSDN SYEWNKVDPY EREQLRVKME DGEFWMSFRD FIREFTKLEI
CNLTPDALKS RTLRNWNTTF YEGTWRRGST AGGCRNYPAT FWVNPQFKIR
LEEVDDADDY DSRESGCSFL LALMQKHRRR ERRFGRDMET IGFAVYQVPR
ELAGQPVHLK RDFFLANASR AQSEHFINLR EVSNRIRLPP GEYIVVPSTF
EPNKEGDFLL RFFSEKKAGT QELDDQIQAN LPDEKVLSEE EIDDNFKTLF
SKLAGDDMEI SVKELQTILN RIISKHKDLR TNGFSLESCR SMVNLMDRDG
NGKLGLVEFN ILWNRIRNYL TIFRKFDLDK SGSMSAYEMR MAIEAAGFKL
NKKLHELIIT RYSEPDLAVD FDNFVCCLVR LETMFRFFKI LDTDLDGVVT
FDLFKWLQLT MFA
Structural Information
- Known structures with covalent ligands:
- Protein structure:
Related Pathway
- Protein processing in endoplasmic reticulum
- Apoptosis
- Necroptosis
- Cellular senescence
- Alzheimer disease
Experimental Evidence
- Crystallography
Reference
- Moldoveanu T, Campbell R L, Cuerrier D, et al. Crystal structures of calpain–E64 and–leupeptin inhibitor complexes reveal mobile loops gating the active site[J]. Journal of molecular biology, 2004, 343(5): 1313-1326. 15491615