Difference between revisions of "Ubiquitin-conjugating enzyme E2 L3"
(Created page with "{| align="left" | __TOC__ |} {{#invoke:InfoboxforTarget|run|UbcH7, UBE2L3, Ubiquitin-protein ligase L3|[https://www.uniprot.org/uniprot/P68036 P68036]|Homo sapiens|Cys86|[...") |
(→Reference) |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
| __TOC__ | | __TOC__ | ||
|} | |} | ||
| − | {{#invoke:InfoboxforTarget|run|UbcH7, UBE2L3, Ubiquitin-protein ligase L3|[https://www.uniprot.org/uniprot/P68036 P68036]|Homo sapiens|Cys86|[http://pfam.xfam.org/family/PF00179 Ubiquitin-conjugating enzyme family]|[[:Category:Ubiquitin-conjugating enzyme E2 L3|Ligand list]]|Ubiquitinase/Deubiquitinase}} | + | {{#invoke:InfoboxforTarget|run|UbcH7, UBE2L3, Ubiquitin-protein ligase L3|[https://www.uniprot.org/uniprot/P68036 P68036]|Homo sapiens|Cys86|[http://pfam.xfam.org/family/PF00179 Ubiquitin-conjugating enzyme family]|[[:Category:Ubiquitin-conjugating enzyme E2 L3|Ligand list]]|Ubiquitinase/Deubiquitinase,<br/>Post-translational Modification}} |
==Summary== | ==Summary== | ||
===Protein Function === | ===Protein Function === | ||
Ubiquitin-conjugating enzyme E2 that specifically acts with HECT-type and RBR family E3 ubiquitin-protein ligases. Does not function with most RING-containing E3 ubiquitin-protein ligases because it lacks intrinsic E3-independent reactivity with lysine: in contrast, it has activity with the RBR family E3 enzymes, such as PRKN and ARIH1, that function like function like RING-HECT hybrids. Accepts ubiquitin from the E1 complex and catalyzes its covalent attachment to other proteins. In vitro catalyzes 'Lys-11'-linked polyubiquitination. Involved in the selective degradation of short-lived and abnormal proteins. Down-regulated during the S-phase it is involved in progression through the cell cycle. Regulates nuclear hormone receptors transcriptional activity. May play a role in myelopoiesis. (From Uniprot)<br/> | Ubiquitin-conjugating enzyme E2 that specifically acts with HECT-type and RBR family E3 ubiquitin-protein ligases. Does not function with most RING-containing E3 ubiquitin-protein ligases because it lacks intrinsic E3-independent reactivity with lysine: in contrast, it has activity with the RBR family E3 enzymes, such as PRKN and ARIH1, that function like function like RING-HECT hybrids. Accepts ubiquitin from the E1 complex and catalyzes its covalent attachment to other proteins. In vitro catalyzes 'Lys-11'-linked polyubiquitination. Involved in the selective degradation of short-lived and abnormal proteins. Down-regulated during the S-phase it is involved in progression through the cell cycle. Regulates nuclear hormone receptors transcriptional activity. May play a role in myelopoiesis. (From Uniprot)<br/> | ||
| − | S-ubiquitinyl-[E1 | + | UBE2L3 expressed in 226 organ(s), highest expression level in C1 segment of cervical spinal cord. <br/> |
| + | The reaction of UBE2L3: <br/> | ||
| + | <div align="center">S-ubiquitinyl-[E1]-L-cysteine + [E2]-L-cysteine = [E1]-L-cysteine + S-ubiquitinyl-[E2]-L-cysteine.</div> | ||
| + | [[File:571-function-UB.jpg|center|1000px]] | ||
| + | <div align="center">PMID: 27002218</div> | ||
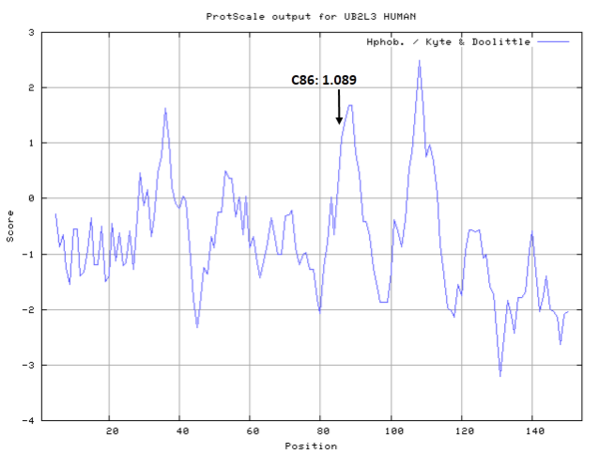
===Cys Function & Property=== | ===Cys Function & Property=== | ||
| Line 42: | Line 46: | ||
# Strickson S, Campbell D G, Emmerich C H, et al. '''The anti-inflammatory drug BAY 11-7082 suppresses the MyD88-dependent signalling network by targeting the ubiquitin system[J].''' Biochemical Journal, 2013, 451(3): 427-437. [https://www.ncbi.nlm.nih.gov/pubmed/?term=23441730 23441730]<br/> | # Strickson S, Campbell D G, Emmerich C H, et al. '''The anti-inflammatory drug BAY 11-7082 suppresses the MyD88-dependent signalling network by targeting the ubiquitin system[J].''' Biochemical Journal, 2013, 451(3): 427-437. [https://www.ncbi.nlm.nih.gov/pubmed/?term=23441730 23441730]<br/> | ||
| − | [[Category: | + | [[Category:Targets]] |
| − | [[Category: | + | [[Category:Homo sapiens]] |
| − | [[Category: | + | [[Category:Ubiquitinase/Deubiquitinase]] |
| − | [[Category: | + | [[Category:Post-translational Modification]] |
| − | [[Category: | + | [[Category:Ubiquitin-conjugating enzyme family]] |
| − | [[Category: | + | [[Category:Ubiquitin mediated proteolysis]] |
| + | [[Category:Parkinson disease]] | ||
Latest revision as of 23:02, 19 August 2019
| Basic Information | |
|---|---|
| Short Name | UbcH7, UBE2L3, Ubiquitin-protein ligase L3 |
| UNP ID | P68036 |
| Organism | Homo sapiens |
| Cys Site | Cys86 |
| Family/Domain | Ubiquitin-conjugating enzyme family |
| Known Ligand | Ligand list |
| Function Type |
Ubiquitinase/Deubiquitinase, Post-translational Modification |
Summary
Protein Function
Ubiquitin-conjugating enzyme E2 that specifically acts with HECT-type and RBR family E3 ubiquitin-protein ligases. Does not function with most RING-containing E3 ubiquitin-protein ligases because it lacks intrinsic E3-independent reactivity with lysine: in contrast, it has activity with the RBR family E3 enzymes, such as PRKN and ARIH1, that function like function like RING-HECT hybrids. Accepts ubiquitin from the E1 complex and catalyzes its covalent attachment to other proteins. In vitro catalyzes 'Lys-11'-linked polyubiquitination. Involved in the selective degradation of short-lived and abnormal proteins. Down-regulated during the S-phase it is involved in progression through the cell cycle. Regulates nuclear hormone receptors transcriptional activity. May play a role in myelopoiesis. (From Uniprot)
UBE2L3 expressed in 226 organ(s), highest expression level in C1 segment of cervical spinal cord.
The reaction of UBE2L3:
Cys Function & Property
Cys87 is the active site of UBE2N, which could form a glycyl thioester intermediate during the catalysis.
- Hydrophobic property:
- SASA:
- Cys87: 32.715 A^2
Protein Sequence
MAASRRLMKE LEEIRKCGMK NFRNIQVDEA NLLTWQGLIV PDNPPYDKGA
FRIEINFPAE YPFKPPKITF KTKIYHPNID EKGQVCLPVI SAENWKPATK
TDQVIQSLIA LVNDPQPEHP LRADLAEEYS KDRKKFCKNA EEFTKKYGEK
RPVD
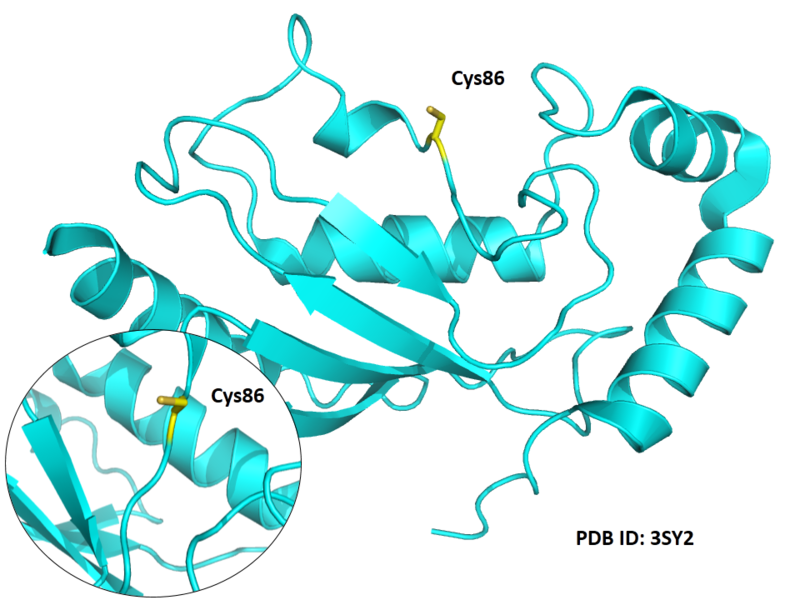
Structural Information
- Known structures with covalent ligands:
- Unknown
- Protein structure:
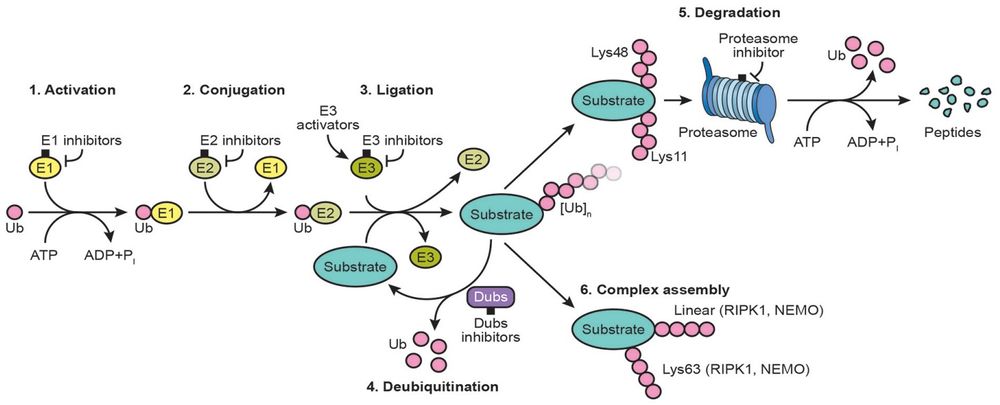
Related Pathway
Experimental Evidence
- MALDI-TOF/MS, Tryptic Digest
Reference
- Strickson S, Campbell D G, Emmerich C H, et al. The anti-inflammatory drug BAY 11-7082 suppresses the MyD88-dependent signalling network by targeting the ubiquitin system[J]. Biochemical Journal, 2013, 451(3): 427-437. 23441730