Difference between revisions of "Pro-cathepsin H"
(Created page with "{| align="left" | __TOC__ |} {{#invoke:InfoboxforTarget|run|CTSH|[https://www.uniprot.org/uniprot/P09668 P09668]|Homo sapiens|Cys141|[http://pfam.xfam.org/family/PF00112 P...") |
(→Reference) |
||
| Line 46: | Line 46: | ||
[[Category:Targets|Targets]] | [[Category:Targets|Targets]] | ||
| + | [[Category:Homo sapiens|Homo sapiens]] | ||
[[Category:Peptidase C1 family|Peptidase C1 family]] | [[Category:Peptidase C1 family|Peptidase C1 family]] | ||
[[Category:Lysosome|Lysosome]] | [[Category:Lysosome|Lysosome]] | ||
Revision as of 20:25, 28 July 2019
Lua error: data must be either of type string or number.
Summary
Protein Function
Cathepsin H is a lysosomal cysteine proteinase, belongs to the peptidase C1 protein family. It is composed of a dimer of disulfide-linked heavy and light chains, both produced from a single protein precursor. The protein can act both as an aminopeptidase and as an endopeptidase. Increased expression of this gene has been correlated with malignant progression of prostate tumors. Two transcript variants encoding different isoforms have been found for this gene. (From Wikipedia)
Important for the overall degradation of proteins in lysosomes. Hydrolysis of proteins, acting as an aminopeptidase (notably, cleaving Arg-|-Xaa bonds) as well as an endopeptidase. (From Uniprot)
Cys Function & Property
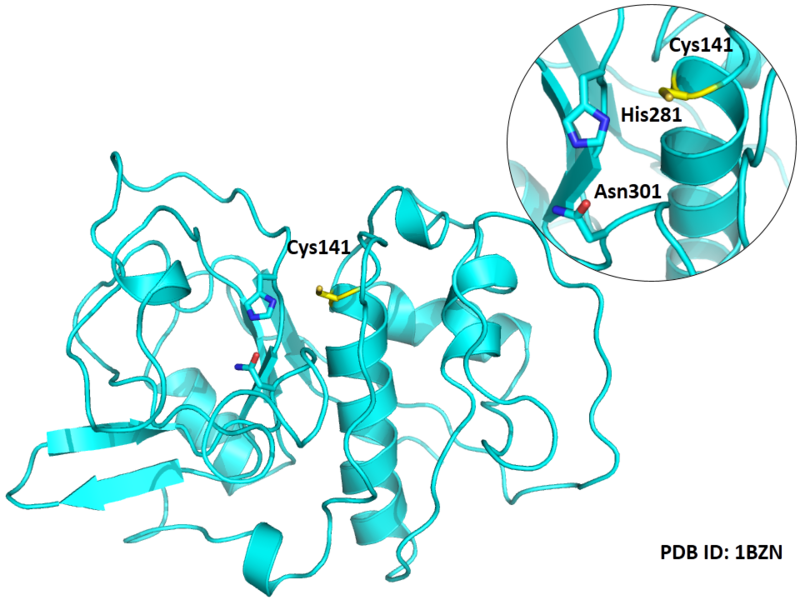
Cys141 is one of the active sites of Cathepsin H, which is very close to His281 and Asn301 in space. These three residues formed a typical catalytic triad motif.
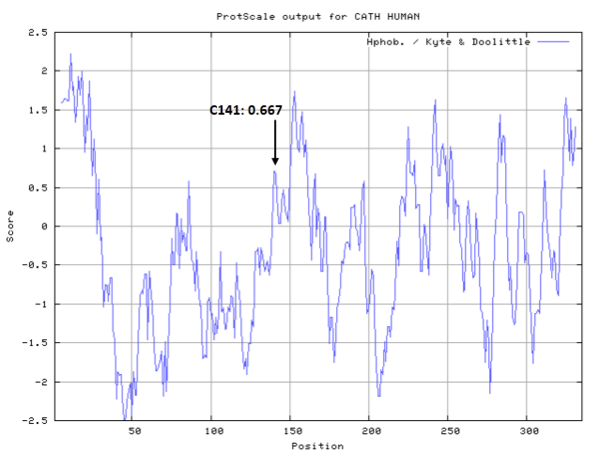
- Hydrophobic property:
- SASA:
- Cys141: 1.32 A^2
Protein Sequence
MWATLPLLCA GAWLLGVPVC GAAELCVNSL EKFHFKSWMS KHRKTYSTEE
YHHRLQTFAS NWRKINAHNN GNHTFKMALN QFSDMSFAEI KHKYLWSEPQ
NCSATKSNYL RGTGPYPPSV DWRKKGNFVS PVKNQGACGS CWTFSTTGAL
ESAIAIATGK MLSLAEQQLV DCAQDFNNHG CQGGLPSQAF EYILYNKGIM
GEDTYPYQGK DGYCKFQPGK AIGFVKDVAN ITIYDEEAMV EAVALYNPVS
FAFEVTQDFM MYRTGIYSST SCHKTPDKVN HAVLAVGYGE KNGIPYWIVK
NSWGPQWGMN GYFLIERGKN MCGLAACASY PIPLV
Structural Information
- Known structures with covalent ligands:
- Unknown
- Protein structure:
Related Pathway
Experimental Evidence
- Homologous Analysis of Sequence, Enzymatic Assay
Reference
- Štern I, Schaschke N, Moroder L, et al. Crystal structure of NS-134 in complex with bovine cathepsin B: a two-headed epoxysuccinyl inhibitor extends along the entire active-site cleft[J]. Biochemical Journal, 2004, 381(2): 511-517. 15084146