Difference between revisions of "Dual specificity phosphatase Cdc25B"
| Line 2: | Line 2: | ||
| __TOC__ | | __TOC__ | ||
|} | |} | ||
| − | {{#invoke:InfoboxforTarget|run|CDC25B, M-phase inducer phosphatase 2|[https://www.uniprot.org/uniprot/P30305 P30305]|Homo sapiens|Cys440, Cys486|[http://pfam.xfam.org/family/PF06617 M-phase inducer (MPI) phosphatase family]|[[:Category:Dual specificity phosphatase Cdc25B|Ligand list]]| | + | {{#invoke:InfoboxforTarget|run|CDC25B, M-phase inducer phosphatase 2|[https://www.uniprot.org/uniprot/P30305 P30305]|Homo sapiens|Cys440, Cys486|[http://pfam.xfam.org/family/PF06617 M-phase inducer (MPI) phosphatase family]|[[:Category:Dual specificity phosphatase Cdc25B|Ligand list]]|Phosphatase}} |
==Summary== | ==Summary== | ||
Revision as of 15:11, 1 August 2019
| Basic Information | |
|---|---|
| Short Name | CDC25B, M-phase inducer phosphatase 2 |
| UNP ID | P30305 |
| Organism | Homo sapiens |
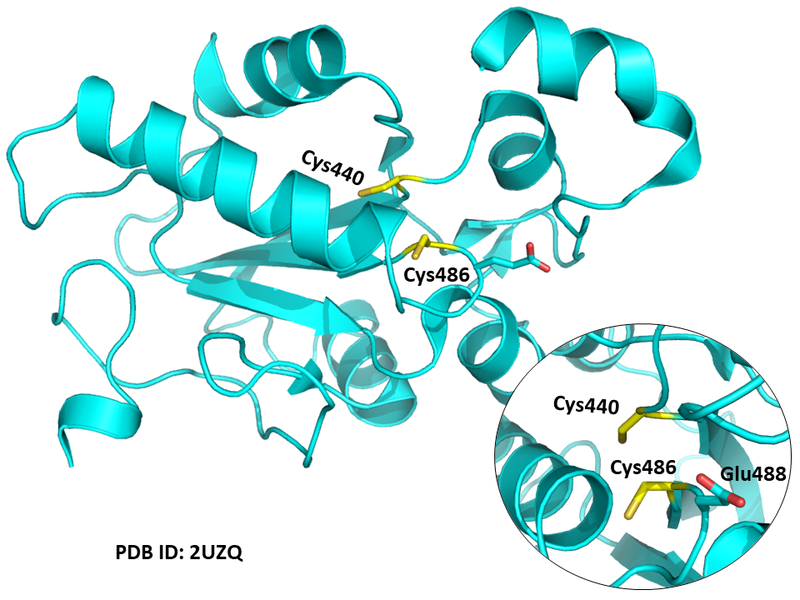
| Cys Site | Cys440, Cys486 |
| Family/Domain | M-phase inducer (MPI) phosphatase family |
| Known Ligand | Ligand list |
| Function Type | Phosphatase |
Summary
Protein Function
M-phase inducer phosphatase 2 is an enzyme that in humans is encoded by the CDC25B gene.
CDC25B is a member of the CDC25 family of phosphatases. CDC25B activates the cyclin dependent kinase CDC2 by removing two phosphate groups and it is required for entry into mitosis. CDC25B shuttles between the nucleus and the cytoplasm due to nuclear localization and nuclear export signals. The protein is nuclear in the M and G1 phases of the cell cycle and moves to the cytoplasm during S and G2. CDC25B has oncogenic properties, although its role in tumor formation has not been determined. Multiple transcript variants for this gene exist. (From Wikipedia)
Cys Function & Property
Cys486 is the active site of CDC25B, and Cys440 is very close to this site in space.
- Hydrophobic property:
- SASA:
- Cys440: 0.411 A^2
- Cys486: 2.207 A^2
Protein Sequence
MEVPQPEPAP GSALSPAGVC GGAQRPGHLP GLLLGSHGLL GSPVRAAASS
PVTTLTQTMH DLAGLGSETP KSQVGTLLFR SRSRLTHLSL SRRASESSLS
SESSESSDAG LCMDSPSPMD PHMAEQTFEQ AIQAASRIIR NEQFAIRRFQ
SMPVRLLGHS PVLRNITNSQ APDGRRKSEA GSGAASSSGE DKENDGFVFK
MPWKPTHPSS THALAEWASR REAFAQRPSS APDLMCLSPD RKMEVEELSP
LALGRFSLTP AEGDTEEDDG FVDILESDLK DDDAVPPGME SLISAPLVKT
LEKEEEKDLV MYSKCQRLFR SPSMPCSVIR PILKRLERPQ DRDTPVQNKR
RRSVTPPEEQ QEAEEPKARV LRSKSLCHDE IENLLDSDHR ELIGDYSKAF
LLQTVDGKHQ DLKYISPETM VALLTGKFSN IVDKFVIVDC RYPYEYEGGH
IKTAVNLPLE RDAESFLLKS PIAPCSLDKR VILIFHCEFS SERGPRMCRF
IRERDRAVND YPSLYYPEMY ILKGGYKEFF PQHPNFCEPQ DYRPMNHEAF
KDELKTFRLK TRSWAGERSR RELCSRLQDQ
Structural Information
- Known structure with covalent ligand:
- Protein structure:
Related Pathway
Experimental Evidence
- Crystallography, MALDI-TOF/MS, Western Blot, Endoproteinase Lys-C Digest, Tryptic Digest
Reference
- Reynolds R A, Yem A W, Wolfe C L, et al. Crystal structure of the catalytic subunit of Cdc25B required for G2/M phase transition of the cell cycle[J]. Journal of molecular biology, 1999, 293(3): 559-568. 10543950
- Tsuchiya A, Asanuma M, Hirai G, et al. CDC25A-inhibitory RE derivatives bind to pocket adjacent to the catalytic site[J]. Molecular BioSystems, 2013, 9(5): 1026-1034. 23467652
- Ueda K, Usui T, Nakayama H, et al. 4‐Isoavenaciolide covalently binds and inhibits VHR, a dual‐specificity phosphatase[J]. FEBS letters, 2002, 525(1-3): 48-52. 12163160