Difference between revisions of "Histone acetyltransferase KAT2B"
(Created page with "{| align="left" | __TOC__ |} {{#invoke:InfoboxforTarget|run|KAT2B, PCAF|[https://www.uniprot.org/uniprot/Q92831 Q92831]|Homo sapiens|Cys574|[http://pfam.xfam.org/family/PF...") |
(No difference)
|
Revision as of 03:02, 15 August 2019
| Basic Information | |
|---|---|
| Short Name | KAT2B, PCAF |
| UNP ID | Q92831 |
| Organism | Homo sapiens |
| Cys Site | Cys574 |
| Family/Domain |
Acetyltransferase family, GCN5 subfamily |
| Known Ligand | Ligand list |
| Function Type | Transcription Related, Post-translational Modification |
Summary
Protein Function
K(lysine) acetyltransferase 2B (KAT2B), also known as P300/CBP-associated factor (PCAF), is a human gene and transcriptional coactivator associated with p53.
CBP and p300 are large nuclear proteins that bind to many sequence-specific factors involved in cell growth and/or differentiation, including c-jun and the adenoviral oncoprotein E1A. The protein encoded by the PCAF gene associates with p300/CBP. It has in vitro and in vivo binding activity with CBP and p300, and competes with E1A for binding sites in p300/CBP. It has histone acetyl transferase activity with core histones and nucleosome core particles, indicating that this protein plays a direct role in transcriptional regulation. (From Wikipedia)
Cys Function & Property
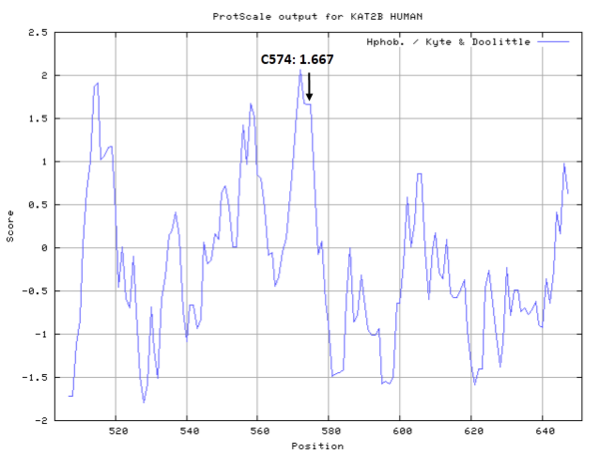
Cys574 is close to the active site Glu570 of KAT2B, which act as the nucleophile group in catalyze.
- Hydrophobic property:
- SASA:
- Cys274: 7.407 A^2
Protein Sequence
MSEAGGAGPG GCGAGAGAGA GPGALPPQPA ALPPAPPQGS PCAAAAGGSG
ACGPATAVAA AGTAEGPGGG GSARIAVKKA QLRSAPRAKK LEKLGVYSAC
KAEESCKCNG WKNPNPSPTP PRADLQQIIV SLTESCRSCS HALAAHVSHL
ENVSEEEMNR LLGIVLDVEY LFTCVHKEED ADTKQVYFYL FKLLRKSILQ
RGKPVVEGSL EKKPPFEKPS IEQGVNNFVQ YKFSHLPAKE RQTIVELAKM
FLNRINYWHL EAPSQRRLRS PNDDISGYKE NYTRWLCYCN VPQFCDSLPR
YETTQVFGRT LLRSVFTVMR RQLLEQARQE KDKLPLEKRT LILTHFPKFL
SMLEEEVYSQ NSPIWDQDFL SASSRTSQLG IQTVINPPPV AGTISYNSTS
SSLEQPNAGS SSPACKASSG LEANPGEKRK MTDSHVLEEA KKPRVMGDIP
MELINEVMST ITDPAAMLGP ETNFLSAko RDEAARLEER RGVIEFHVVG
NSLNQKPNKK ILMWLVGLQN VFSHQLPRMP KEYITRLVFD PKHKTLALIK
DGRVIGGICF RMFPSQGFTE IVFCAVTSNE QVKGYGTHLM NHLKEYHIKH
DILNFLTYAD EYAIGYFKKQ GFSKEIKIPK TKYVGYIKDY EGATLMGCEL
NPRIPYTEFS VIIKKQKEII KKLIERKQAQ IRKVYPGLSC FKDGVRQIPI
ESIPGIRETG WKPSGKEKSK EPRDPDQLYS TLKSILQQVK SHQSAWPFME
PVKRTEAPGY YEVIRFPMDL KTMSERLKNR YYVSKKLFMA DLQRVFTNCK
EYNPPESEYY KCANILEKFF FSKIKEAGLI DK
Structural Information
- Known structure with covalent ligand:
- Unknown
- Protein structure:
Related Pathway
- Notch signaling pathway
- Thyroid hormone signaling pathway
- Human T-cell leukemia virus 1 infection
- Viral carcinogenesis
Experimental Evidence
- LC/MS, Homologous Analysis of Sequence, Enzymatic Assay, Molecular Docking
Reference
- Stimson L, Rowlands M G, Newbatt Y M, et al. Isothiazolones as inhibitors of PCAF and p300 histone acetyltransferase activity[J]. Molecular cancer therapeutics, 2005, 4(10): 1521-1532. 16227401
- Wisastra R, Ghizzoni M, Maarsingh H, et al. Isothiazolones; thiol-reactive inhibitors of cysteine protease cathepsin B and histone acetyltransferase PCAF[J]. Organic & biomolecular chemistry, 2011, 9(6): 1817-1822. 21267493
- Dekker F J, Ghizzoni M, van der Meer N, et al. Inhibition of the PCAF histone acetyl transferase and cell proliferation by isothiazolones[J]. Bioorganic & medicinal chemistry, 2009, 17(2): 460-466. 19111471