DNA repair protein RAD51 homolog 1
| Basic Information | |
|---|---|
| Short Name | hRAD51, RAD51 |
| UNP ID | Q06609 |
| Organism | Homo sapiens |
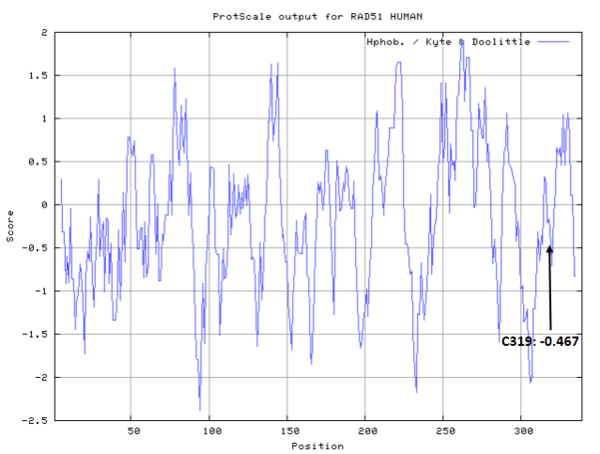
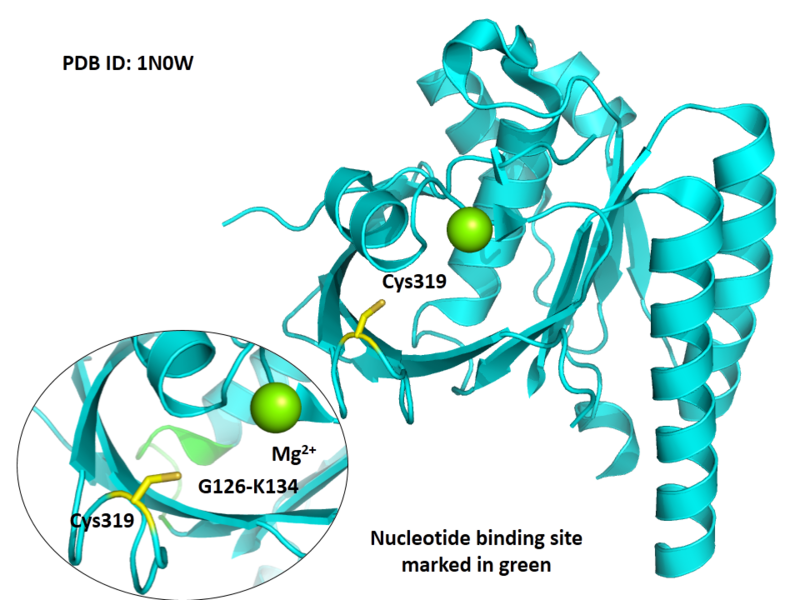
| Cys Site | Cys319 |
| Family/Domain |
RecA family, RAD51 subfamily |
| Known Ligand | Ligand list |
| Function Type | DNA repair enzyme |
Summary
Protein Function
RAD51 plays an important role in homologous strand exchange, a key step in DNA repair through homologous recombination (HR) (PMID: 28575658). Binds to single and double-stranded DNA and exhibits DNA-dependent ATPase activity. Catalyzes the recognition of homology and strand exchange between homologous DNA partners to form a joint molecule between a processed DNA break and the repair template. Binds to single-stranded DNA in an ATP-dependent manner to form nucleoprotein filaments which are essential for the homology search and strand exchange (PMID: 26681308). Part of a PALB2-scaffolded HR complex containing BRCA2 and RAD51C and which is thought to play a role in DNA repair by HR. Plays a role in regulating mitochondrial DNA copy number under conditions of oxidative stress in the presence of RAD51C and XRCC3. Also involved in interstrand cross-link repair (PMID: 26253028). (From Uniprot)
Cys Function & Property
Cys319 is very close to the ATP and metal ion binding site in space.
- Hydrophobic property:
- SASA:
- Cys319: 105.372 A^2
Protein Sequence
MAMQMQLEAN ADTSVEEESF GPQPISRLEQ CGINANDVKK LEEAGFHTVE
AVAYAPKKEL INIKGISEAK ADKILAEAAK LVPMGFTTAT EFHQRRSEII
QITTGSKELD KLLQGGIETG SITEMFGEFR TGKTQICHTL AVTCQLPIDR
GGGEGKAMYI DTEGTFRPER LLAVAERYGL SGSDVLDNVA YARAFNTDHQ
TQLLYQASAM MVESRYALLI VDSATALYRT DYSGRGELSA RQMHLARFLR
MLLRLADEFG VAVVITNQVV AQVDGAAMFA ADPKKPIGGN IIAHASTTRL
YLRKGRGETR ICKIYDSPCL PEAEAMFAIN ADGVGDAKD
Structural Information
- Known structure with covalent ligand:
- Unknown
- Protein structure:
Related Pathway
Experimental Evidence
- Fluorescence Polarization Assay, Cys-directed Mutation
Reference
- Budke B, Kalin J H, Pawlowski M, et al. An optimized RAD51 inhibitor that disrupts homologous recombination without requiring Michael acceptor reactivity[J]. Journal of medicinal chemistry, 2012, 56(1): 254-263. 23231413
- Budke B, Logan H L, Kalin J H, et al. RI-1: a chemical inhibitor of RAD51 that disrupts homologous recombination in human cells[J]. Nucleic acids research, 2012, 40(15): 7347-7357. 22573178