Caspase-6
| Basic Information | |
|---|---|
| Short Name | CASP6 |
| UNP ID | P55212 |
| Organism | Homo sapiens |
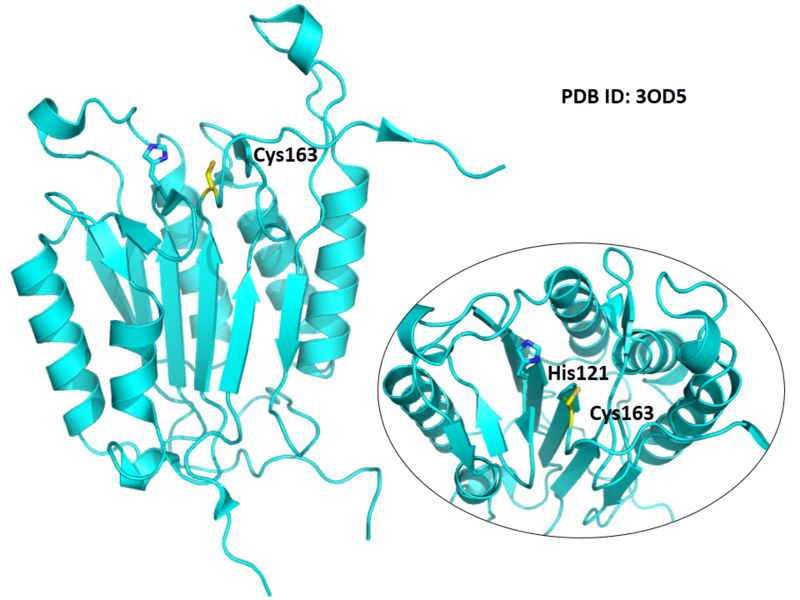
| Cys Site | Cys163 |
| Family/Domain | Peptidase C14A family |
| Known Ligand | Ligand list |
| Function Type | Protease |
Summary
Protein Function
Caspase-6 is a member of the cysteine-aspartic acid protease (caspase) family. Sequential activation of caspases plays a central role in the execution-phase of cell apoptosis. Caspases exist as inactive proenzymes that undergo proteolytic processing at conserved aspartic residues to produce two subunits, large and small, that dimerize to form the active enzyme. This protein is processed by caspases-7, -8 and -10, and is thought to function as a downstream enzyme in the caspase activation cascade.
Caspase-6 can also undergo self-processing without other members of the caspase family. Alternative splicing of this gene results in two transcript variants that encode different isoforms. (From Wikipedia)
Cys Function & Property
The catalytic triad in Caspase-6 comprises Cys163, His121 and Arg64.
- Hydrophobic property:
- SASA:
- Cys163: 60.255 A^2
Protein Sequence
MSSASGLRRG HPAGGEENMT ETDAFYKREM FDPAEKYKMD HRRRGIALIF
NHERFFWHLT LPERRGTCAD RDNLTRRFSD LGFEVKCFND LKAEELLLKI
HEVSTVSHAD ADCFVCVFLS HGEGNHIYAY DAKIEIQTLT GLFKGDKCHS
LVGKPKIFII QACRGNQHDV PVIPLDVVDN QTEKLDTNIT EVDAASVYTL
PAGADFLMCY SVAEGYYSHR ETVNGSWYIQ DLCEMLGKYG SSLEFTELLT
LVNRKVSQRR VDFCKDPSAI GKKQVPCFAS MLTKKLHFFP KSN
Structural Information
- Known structure with covalent ligand:
- Protein structure:
Related Pathway
Experimental Evidence
- Crystallography, Site-directed mutation assasy
Reference
- Müller I, Lamers M B A C, Ritchie A J, et al. Structure of human caspase-6 in complex with Z-VAD-FMK: new peptide binding mode observed for the non-canonical caspase conformation[J]. Bioorganic & medicinal chemistry letters, 2011, 21(18): 5244-5247. 21820899
- Liu X, Zhang H, Wang X J, et al. Get phases from arsenic anomalous scattering: de novo SAD phasing of two protein structures crystallized in cacodylate buffer[J]. PLoS One, 2011, 6(9): e24227. 21912678