Hsp90 co-chaperone Cdc37
| Basic Information | |
|---|---|
| Short Name | CDC37 |
| UNP ID | Q16543 |
| Organism | Homo sapiens |
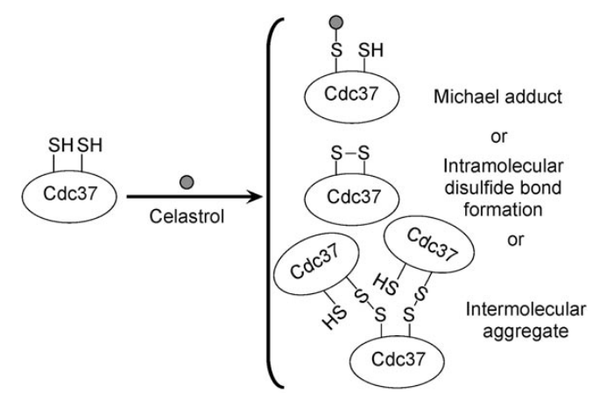
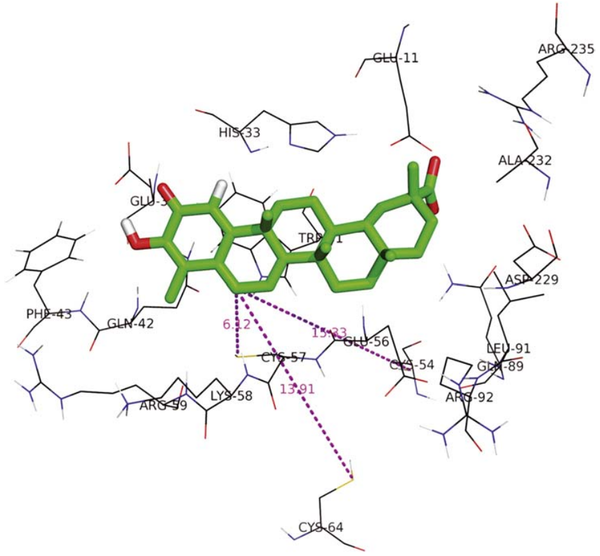
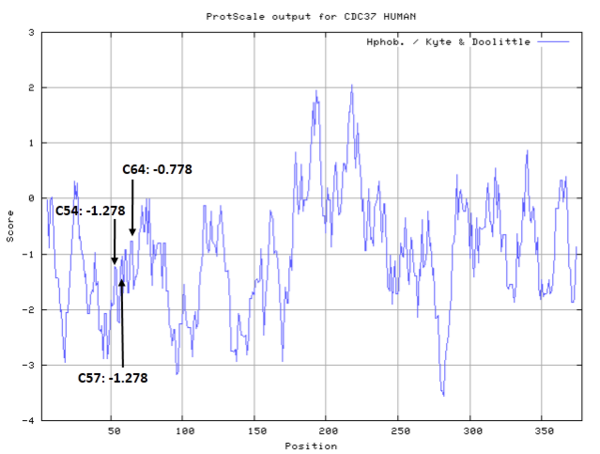
| Cys Site | Cys57, Cys54, Cys64 |
| Family/Domain | CDC37 family |
| Known Ligand | Ligand list |
| Function Type | Chaperone |
Summary
Protein Function
Hsp90 co-chaperone Cdc37 is a molecular chaperone with specific function in cell signal transduction. It has been shown to form complex with Hsp90 and a variety of protein kinases including CDK4, CDK6, SRC, RAF1, MOK, as well as eIF-2 alpha kinases, and is upregulated in various cancers. It is thought to play a critical role in directing Hsp90 to its target kinases. The protein–protein complex forms with a KD value of 1.2 mm and is considered to mediate carcinogenesis by stabilizing a variety of different oncogenic kinases in malignant cells.
CDC37 consists of three structural domains. The N-terminal domain binds to protein kinases. The central domain is the Hsp90chaperone (heat shock protein 90) binding domain. The function of the C-terminal domain is unclear. (From Uniprot, Wikipedia, PMID: 19585625)
Cys Function & Property
From the mutation, homology modeling and other assay, Cys57, Cys54, Cys64 are confirmed to located in/near the celastrol binding pocket.
- Hydrophobic property:
- SASA:
- Unknown
Protein Sequence
MVDYSVWDHI EVSDDEDETH PNIDTASLFR WRHQARVERM EQFQKEKEEL
DRGCRECKRK VAECQRKLKE LEVAEGGKAE LERLQAEAQQ LRKEERSWEQ
KLEEMRKKEK SMPWNVDTLS KDGFSKSMVN TKPEKTEEDS EEVREQKHKT
FVEKYEKQIK HFGMLRRWDD SQKYLSDNVH LVCEETANYL VIWCIDLEVE
EKCALMEQVA HQTIVMQFIL ELAKSLKVDP RACFRQFFTK IKTADRQYME
GFNDELEAFK ERVRGRAKLR IEKAMKEYEE EERKKRLGPG GLDPVEVYES
LPEELQKCFD VKDVQMLQDA ISKMDPTDAK YHMQRCIDSG LWVPNSKASE
AKEGEEAGPG DPLLEAVPKT GDEKDVSV
Structural Information
- Known structure with covalent ligand:
- Unknown
- Protein structure:
- Unknown
Related Pathway
Experimental Evidence
- Nuclear Magnetic Resonance, Mass Spectrometry
Reference
- Sreeramulu S, Gande S L, Göbel M, et al. Molecular mechanism of inhibition of the human protein complex Hsp90–Cdc37, a kinome chaperone–cochaperone, by triterpene celastrol[J]. Angewandte Chemie International Edition, 2009, 48(32): 5853-5855. 19585625
- Duan Y, Jin H, Yu H, et al. Computational investigation of interactions between Cdc37 and celastrol[J]. Molecular Simulation, 2013, 39(4): 270-278. DOI:10.1080/08927022.2012.718439.