Kelch-like ECH-associated protein 1 (Homo sapiens)
| Basic Information | |
|---|---|
| Short Name | Keap1, INrf2 |
| UNP ID | P57790 |
| Organism | Homo sapiens |
| Cys Site |
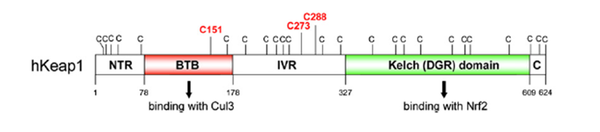
Cys38, Cys151, Cys257, Cys273, Cys288 |
| Family/Domain |
BTB/POZ domain, Kelch motif |
| Known Ligand | Ligand list |
| Function Type | Redox protein |
Summary
Protein Function
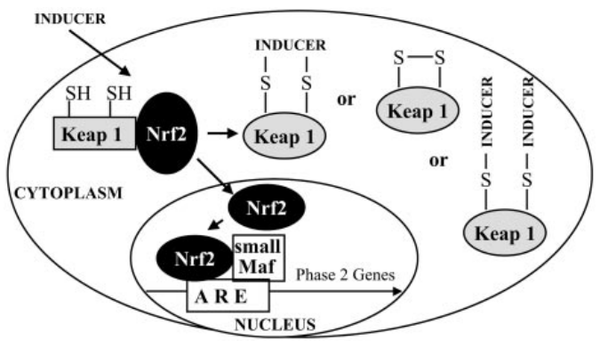
Keap1 (Kelch-like ECH-associated protein 1) is a multi-domain protein which plays a key role in the regulation of Nrf2, a transcription factor that mediates the expression of a large array of cytoprotective enzymes in response to electrophilic and oxidative assault. It acts in concert with members of the CRL3 class of Cullin-RING-Ligase E3 ligases to provide substrate-specific recruitment for ubiquitination, and consists of a three domain architecture composed of an N-terminal BTB (Broad complex, Tramtrack, and Bric-a-Brac) domain, an intervening region (IVR) or BACK domain, and a C-terminal Kelch repeat domain. (PMID: 24896564)
Acts as a substrate adapter protein for the E3 ubiquitin ligase complex formed by CUL3 and RBX1 and targets NFE2L2/NRF2 for ubiquitination and degradation by the proteasome, thus resulting in the suppression of its tran-scriptional activity and the repression of antioxidant response element-mediated detoxifying enzyme gene expression. Retains NFE2L2/NRF2 and may also retain BPTF in the cytosol. Targets PGAM5 for ubiquitination and degradation by the proteasome. (From Uniprot)
Cys Function & Property
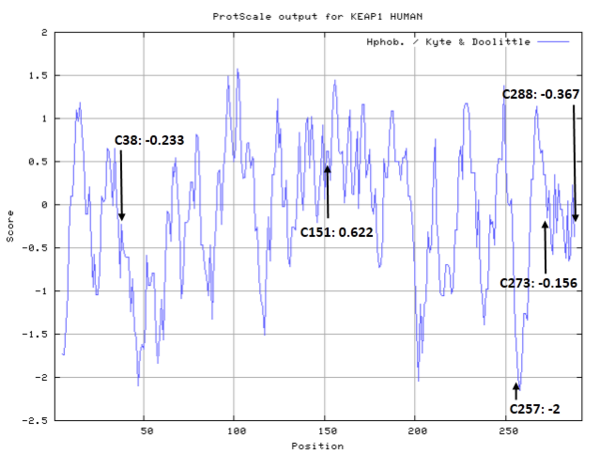
There are 25 cystiene residues in Keap1, and could form multiple disulfide bonds or free thiol groups under different situations (see below).
- Hydrophobic property:
- SASA:
- Cys38: Unknown
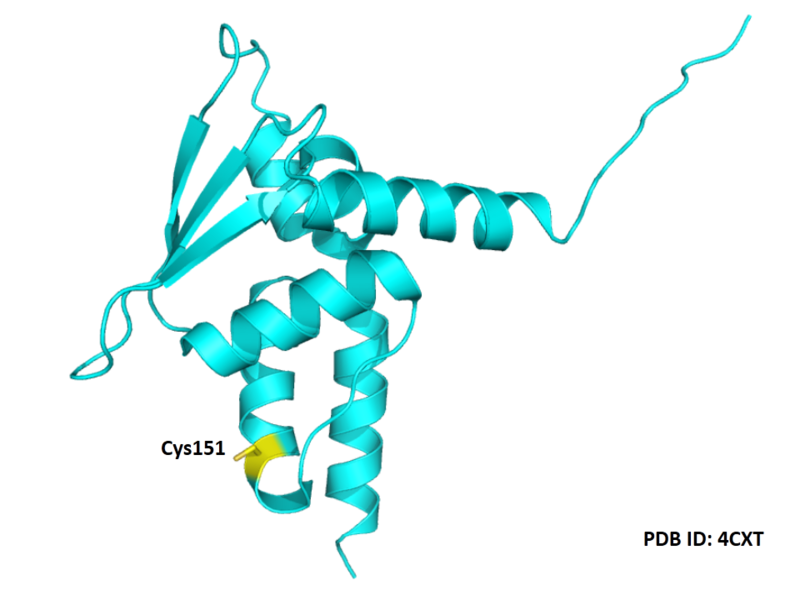
- Cys151: 38.299 A^2
- Cys257: Unknown
- Cys273: Unknown
- Cys288: Unknown
Protein Sequence
MQPDPRPSGA GACCRFLPLQ SQCPEGAGDA VMYASTECKA EVTPSQHGNR
TFSYTLEDHT KQAFGIMNEL RLSQQLCDVT LQVKYQDAPA AQFMAHKVVL
ASSSPVFKAM FTNGLREQGM EVVSIEGIHP KVMERLIEFA YTASISMGEK
CVLHVMNGAV MYQIDSVVRA CSDFLVQQLD PSNAIGIANF AEQIGCVELH
QRAREYIYMH FGEVAKQEEF FNLSHCQLVT LISRDDLNVR CESEVFHACI
NWVKYDCEQR RFYVQALLRA VRCHSLTPNF LQMQLQKCEI LQSDSRCKDY
LVKIFEELTL HKPTQVMPCR APKVGRLIYT AGGYFRQSLS YLEAYNPSDG
TWLRLADLQV PRSGLAGCVV GGLLYAVGGR NNSPDGNTDS SALDCYNPMT
NQWSPCAPMS VPRNRIGVGV IDGHIYAVGG SHGCIHHNSV ERYEPERDEW
HLVAPMLTRR IGVGVAVLNR LLYAVGGFDG TNRLNSAECY YPERNEWRMI
TAMNTIRSGA GVCVLHNCIY AAGGYDGQDQ LNSVERYDVE TETWTFVAPM
KHRRSALGIT VHQGRIYVLG GYDGHTFLDS VECYDPDTDT WSEVTRMTSG
RSGVGVAVTM EPCRKQIDQQ NCTC
Structural Information
- Known structures with covalent ligands:
- Protein structure:
Related Pathway
- Ubiquitin mediated proteolysis
- Pathways in cancer
- Hepatocellular carcinoma
- Fluid shear stress and atherosclerosis
Experimental Evidence
- Crystallography, LC-ESI-MS/MS, Tryptic Digest
Reference
- Copple I M, Goldring C E, Jenkins R E, et al. The hepatotoxic metabolite of acetaminophen directly activates the Keap1‐Nrf2 cell defense system[J]. Hepatology, 2008, 48(4): 1292-1301. 18785192
- Satoh T, Kosaka K, Itoh K, et al. Carnosic acid, a catechol‐type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S‐alkylation of targeted cysteines on Keap1[J]. Journal of neurochemistry, 2008, 104(4): 1116-1131. 17995931