Vitamin D3 receptor
| Basic Information | |
|---|---|
| Short Name | VDR, NR1I1 |
| UNP ID | P11473 |
| Organism | Homo sapiens |
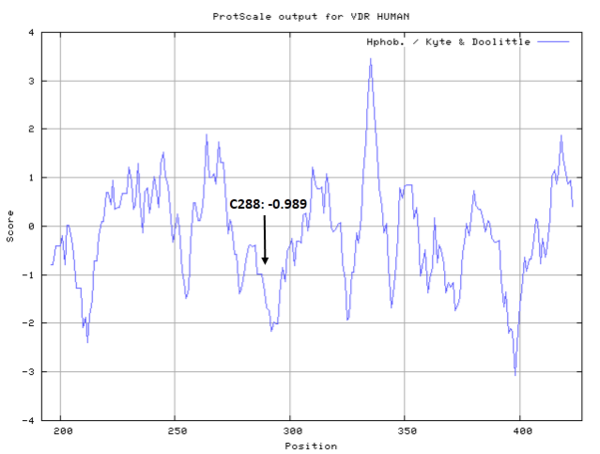
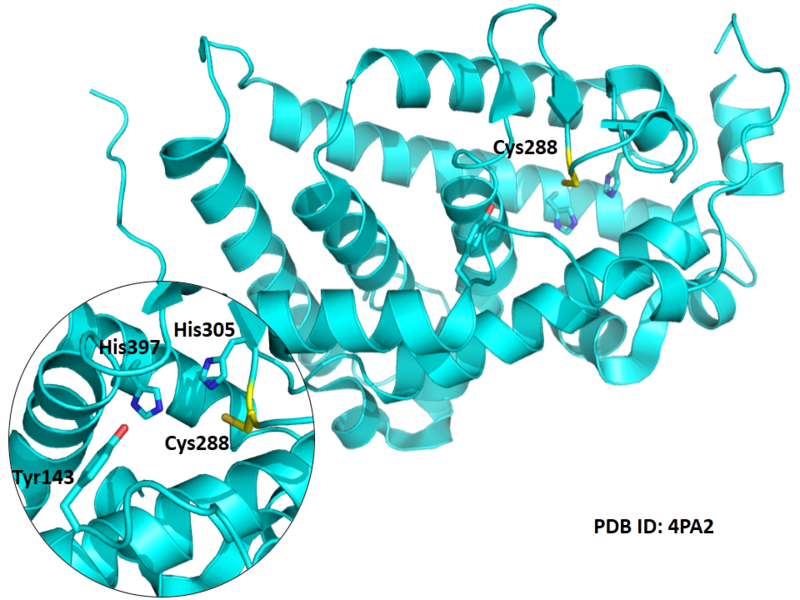
| Cys Site | Cys288 |
| Family/Domain |
Ligand-binding domain of nuclear hormone receptor, Nuclear hormone receptor family, NR1 subfamily |
| Known Ligand | Ligand list |
| Function Type | Transcription Related, Metabolic enzyme |
Summary
Protein Function
Vitamin D receptor (VDR), the nuclear receptor for 1α,25-dihydroxyvitamin D3 [1α,25-(OH)2D3], plays a pivotal role in the manifestation of multiple physiological properties of 1α,25-(OH)2D3, including calcium and phosphorus homeostasis, regulation of the immune response, and modulation of the growth and maturation of normal and malignant cells. 1α,25-(OH)2D3 binds VDR with high specificity and efficiency, allosterically promoting its heterodimerization with retinoid X receptor (RXR). The liganded dimer interacts with vitamin D response elements (VDREs) in the upstream promoter regions of vitamin D-regulated genes to trigger transcription and translation of the gene products. Since binding of 1α,25-(OH)2D3 to VDR must occur to initiate this cascade of physiological responses, analysis of the structure/function aspects of this interaction assumes major significance, especially in light of the fact that more than 500 analogues of 1α,25-(OH)2D3 have been synthesized and physiological effects of many were analyzed both in vitro and in vivo. (PMID: 11015194)
Cys Function & Property
Cys288 is very close to the active site of VDR in space.
- Hydrophobic property:
- SASA:
- Cys288: 1.86 A^2
Protein Sequence
MEAMAASTSL PDPGDFDRNV PRICGVCGDR ATGFHFNAMT CEGCKGFFRR
SMKRKALFTC PFNGDCRITK DNRRHCQACR LKRCVDIGMM KEFILTDEEV
QRKREMILKR KEEEALKDSL RPKLSEEQQR IIAILLDAHH KTYDPTYSDF
CQFRPPVRVN DGGGSHPSRP NSRHTPSFSG DSSSSCSDHC ITSSDMMDSS
SFSNLDLSEE DSDDPSVTLE LSQLSMLPHL ADLVSYSIQK VIGFAKMIPG
FRDLTSEDQI VLLKSSAIEV IMLRSNESFT MDDMSWTCGN QDYKYRVSDV
TKAGHSLELI EPLIKFQVGL KKLNLHEEEH VLLMAICIVS PDRPGVQDAA
LIEAIQDRLS NTLQTYIRCR HPPPGSHLLY AKMIQKLADL RSLNEEHSKQ
YRCLSFQPEC SMKLTPLVLE VFGNEIS
Structural Information
- Known structure with covalent ligand:
- Unknown
- Protein structure:
Related Pathway
- Parathyroid hormone synthesis, secretion and action
- Endocrine and other factor-regulated calcium reabsorption
- Mineral absorption
- Tuberculosis
Experimental Evidence
- Isotope Labeling, Homology Modeling, Molecular Docking
Reference
- Swamy N, Xu W, Paz N, et al. Molecular modeling, affinity labeling, and site-directed mutagenesis define the key points of interaction between the ligand-binding domain of the vitamin D nuclear receptor and 1α, 25-dihydroxyvitamin D3[J]. Biochemistry, 2000, 39(40): 12162-12171. 11015194