Cathepsin C
| Basic Information | |
|---|---|
| Short Name | Dipeptidyl peptidase 1, CTSC, DPP-I |
| UNP ID | P53634 |
| Organism | Homo sapiens |
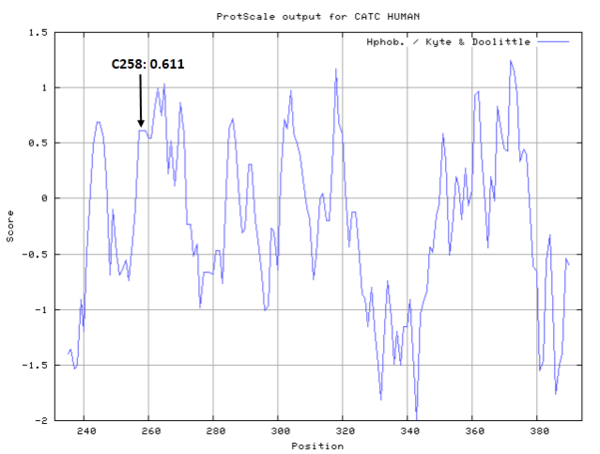
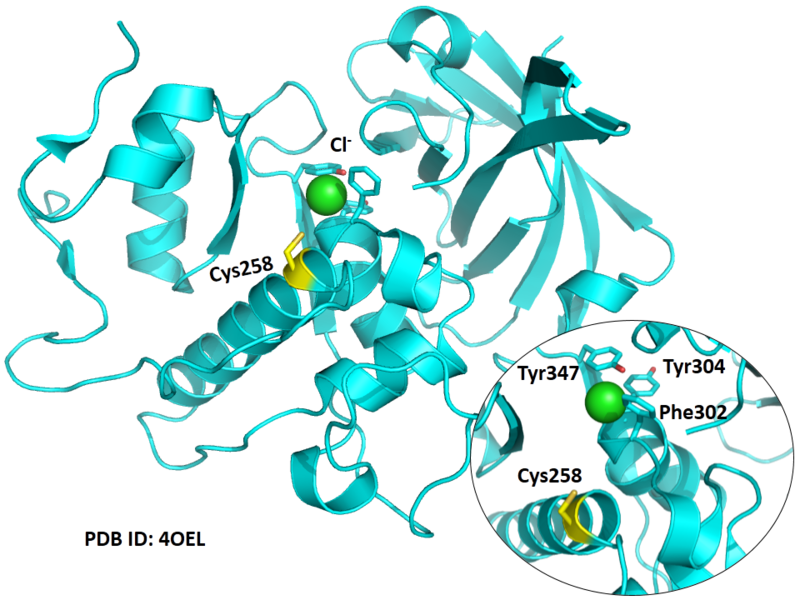
| Cys Site | Cys258 |
| Family/Domain | Peptidase C1 family |
| Known Ligand | Ligand list |
| Function Type | Protease |
Summary
Protein Function
Cathepsin C (CatC or dipeptidyl peptidase I) is a lysosomal cysteine protease which could act as both an exopeptidase and endopeptidase. It is important for intracellular protein degradation and plays a key role in the activation of the proinflammatory serine proteases neutrophil elastase (NE), cathepsin G (CatG), and proteinase-3 (PR-3).
CatC can also activate neuraminidase and factor XIII. In humans, CatC mutations are associated with the autosomal recessive disorders Haim−Munck syndrome and Papillon−Lefèvre syndrome, both associated with almost complete loss of the secondary enzymatic activities of all three serine proteases, NE, CatG, and PR-3, in neutrophils and granzyme B in NK cells and characterized by palmoplantar keratosis and severe childhood periodontal disease. (From Uniprot, PMID: 24592859)
Cys Function & Property
Cys258 is one of the active sites of CatC.
The CGSC sequecne beside Cys258 is very conserved in peptidase family.
- Hydrophobic property:
- SASA:
- Cys258: 10.925 A^2
Protein Sequence
MGAGPSLLLA ALLLLLSGDG AVRCDTPANC TYLDLLGTWV FQVGSSGSQR
DVNCSVMGPQ EKKVVVYLQK LDTAYDDLGN SGHFTIIYNQ GFEIVLNDYK
WFAFFKYKEE GSKVTTYCNE TMTGWVHDVL GRNWACFTGK KVGTASENVY
VNIAHLKNSQ EKYSNRLYKY DHNFVKAINA IQKSWTATTY MEYETLTLGD
MIRRSGGHSR KIPRPKPAPL TAEIQQKILH LPTSWDWRNV HGINFVSPVR
NQASCGSCYS FASMGMLEAR IRILTNNSQT PILSPQEVVS CSQYAQGCEG
GFPYLIAGKY AQDFGLVEEA CFPYTGTDSP CKMKEDCFRY YSSEYHYVGG
FYGGCNEALM KLELVHHGPM AVAFEVYDDF LHYKKGIYHH TGLRDPFNPF
ELTNHAVLLV GYGTDSASGM DYWIVKNSWG TGWGENGYFR IRRGTDECAI
ESIAVAATPI PKL
Structural Information
- Known structure with covalent ligand:
- Protein structure:
Related Pathway
Experimental Evidence
- Molecular Docking, Homologous Analysis of Sequence
Reference
- Mølgaard A, Arnau J, Lauritzen C, et al. The crystal structure of human dipeptidyl peptidase I (cathepsin C) in complex with the inhibitor Gly-Phe-CHN2[J]. Biochemical Journal, 2007, 401(3): 645-650. 17020538
- Furber M, Tiden A K, Gardiner P, et al. Cathepsin C inhibitors: property optimization and identification of a clinical candidate[J]. Journal of medicinal chemistry, 2014, 57(6): 2357-2367. 24592859
- Méthot N, Rubin J, Guay D, et al. Inhibition of the activation of multiple serine proteases with a cathepsin C inhibitor requires sustained exposure to prevent pro-enzyme processing[J]. Journal of Biological Chemistry, 2007, 282(29): 20836-20846. 17535802