Inosine-5'-monophosphate dehydrogenase 1
| Basic Information | |
|---|---|
| Short Name | IMPDH1 |
| UNP ID | P20839 |
| Organism | Homo sapiens |
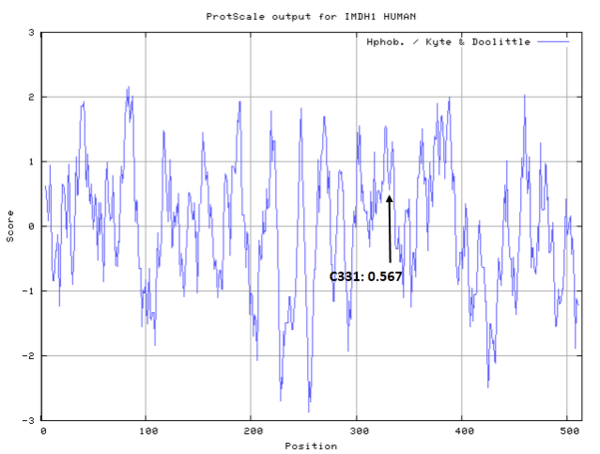
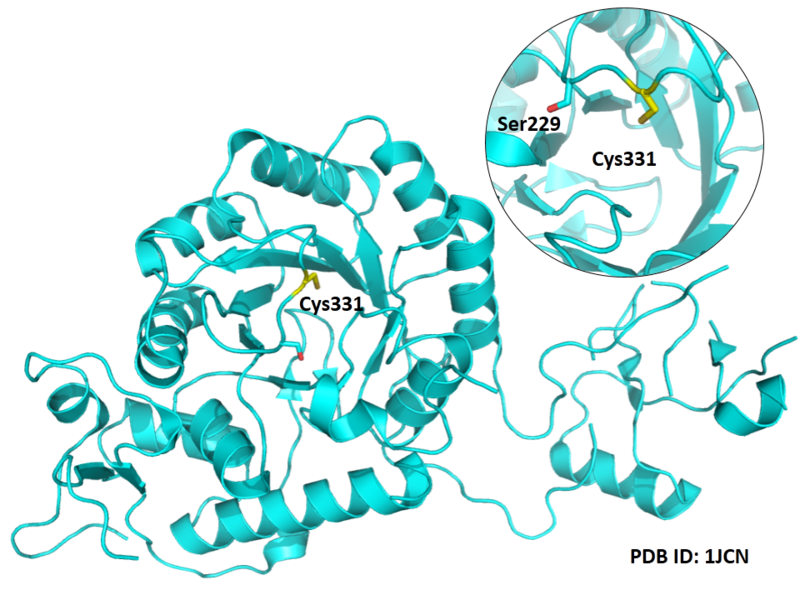
| Cys Site | Cys331 |
| Family/Domain | IMPDH/GMPR family |
| Known Ligand | Ligand list |
| Function Type | Metabolic enzyme |
Summary
Protein Function
IMPDH1 catalyzes the conversion of inosine 5'-phosphate (IMP) to xanthosine 5'-phosphate (XMP), the first committed and rate-limiting step in the de novo synthesis of guanine nucleotides, and therefore plays an important role in the regulation of cell growth. It could also have a single-stranded nucleic acid-binding activity and could play a role in RNA and/or DNA metabolism. It may also have a role in the development of malignancy and the growth progression of some tumors. (From Uniprot)
Cys Function & Property
Cys331 is one of the active sites of IMPDH2, acts as the thioimidate intermediate during the catalytic process.
- Hydrophobic property:
- SASA:
- Cys331: 57.571 A^2
Protein Sequence
MADYLISGGT GYVPEDGLTA QQLFASADGL TYNDFLILPG FIDFIADEVD
LTSALTRKIT LKTPLISSPM DTVTEADMAI AMALMGGIGF IHHNCTPEFQ
ANEVRKVKKF EQGFITDPVV LSPSHTVGDV LEAKMRHGFS GIPITETGTM
GSKLVGIVTS RDIDFLAEKD HTTLLSEVMT PRIELVVAPA GVTLKEANEI
LQRSKKGKLP IVNDCDELVA IIARTDLKKN RDYPLASKDS QKQLLCGAAV
GTREDDKYRL DLLTQAGVDV IVLDSSQGNS VYQIAMVHYI KQKYPHLQVI
GGNVVTAAQA KNLIDAGVDG LRVGMGCGSI CITQEVMACG RPQGTAVYKV
AEYARRFGVP IIADGGIQTV GHVVKALALG ASTVMMGSLL AATTEAPGEY
FFSDGVRLKK YRGMGSLDAM EKSSSSQKRY FSEGDKVKIA QGVSGSIQDK
GSIQKFVPYL IAGIQHGCQD IGARSLSVLR SMMYSGELKF EKRTMSAQIE
GGVHGLHSYE KRLY
Structural Information
- Known structures with covalent ligands:
- Protein structure:
Related Pathway
Experimental Evidence
- Crystallography
Reference
- Balzarini J, Karlsson A, Wang L, et al. Eicar (5-ethynyl-1-beta-D-ribofuranosylimidazole-4-carboxamide). A novel potent inhibitor of inosinate dehydrogenase activity and guanylate biosynthesis[J]. Journal of Biological Chemistry, 1993, 268(33): 24591-24598. 7901217