Protein-arginine deiminase type-1
| Basic Information | |
|---|---|
| Short Name | PADI1, PDI1 |
| UNP ID | Q9ULC6 |
| Organism | Homo sapiens |
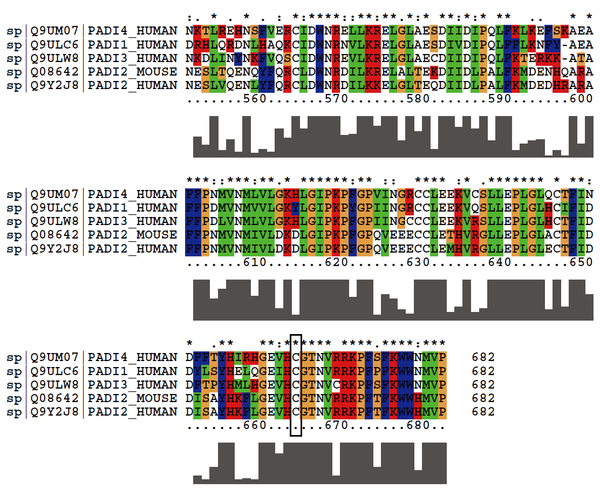
| Cys Site | Cys645 |
| Family/Domain | Protein arginine deiminase family |
| Known Ligand | Ligand list |
| Function Type | Metabolic enzyme |
Summary
Protein Function
Peptidyl arginine deiminase, type I, also known as PADI1, is a protein which in humans is encoded by the PADI1 gene. This gene encodes a member of the peptidyl arginine deiminase family of enzymes, which catalyze the post-translational deimination of proteins by converting arginine residues into citrullines in the presence of calcium ions. The family members have distinct substrate specificities and tissue-specific expression patterns. The type I enzyme is involved in the late stages of epidermal differentiation, where it deiminates filaggrin and keratin K1, which maintains hydration of the stratum corneum, and hence the cutaneous barrier function. This enzyme may also play a role in hair follicle formation. This gene exists in a cluster with four other paralogous genes. (From Wikipedia)
Cys Function & Property
Cys645 is the active site of PADI1.
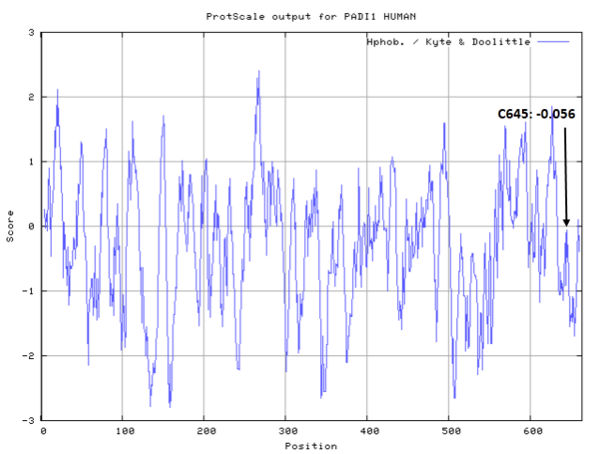
- Hydrophobic property:
- SASA:
- Cys645: Unknown
Protein Sequence
MAPKRVVQLS LKMPTHAVCV VGVEAHVDIH SDVPKGANSF RVSGSSGVEV
FMVYNRTRVK EPIGKARWPL DTDADMVVSV GTASKELKDF KVRVSYFGEQ
EDQALGRSVL YLTGVDISLE VDTGRTGKVK RSQGDKKTWR WGPEGYGAIL
LVNCDRDNHR SAEPDLTHSW LMSLADLQDM SPMLLSCNGP DKLFDSHKLV
LNVPFSDSKR VRVFCARGGN SLSDYKQVLG PQCLSYEVER QPGEQEIKFY
VEGLTFPDAD FLGLVSLSVS LVDPGTLPEV TLFTDTVGFR MAPWIMTPNT
QPPEELYVCR VMDTHGSNEK FLEDMSYLTL KANCKLTICP QVENRNDRWI
QDEMEFGYIE APHKSFPVVF DSPRNRGLKD FPYKRILGPD FGYVTREIPL
PGPSSLDSFG NLDVSPPVTV GGTEYPLGRI LIGSSFPKSG GRQMARAVRN
FLKAQQVQAP VELYSDWLSV GHVDEFLTFV PTSDQKGFRL LLASPSACLK
LFQEKKEEGY GEAAQFDGLK HQAKRSINEM LADRHLQRDN LHAQKCIDWN
RNVLKRELGL AESDIVDIPQ LFFLKNFYAE AFFPDMVNMV VLGKYLGIPK
PYGPIINGRC CLEEKVQSLL EPLGLHCIFI DDYLSYHELQ GEIHCGTNVR
RKPFPFKWWN MVP
Structural Information
- Known structure with covalent ligand:
- Unknown
- Protein structure:
- Unknown
Related Pathway
- Unknown
Experimental Evidence
- Homologous Analysis Of Sequence, Molecular Docking, Cys-directed Mutation, MS
Reference
- Bello A M, Wasilewski E, Wei L, et al. Interrogation of the active sites of protein arginine deiminases (PAD1,-2, and-4) using designer probes[J]. ACS medicinal chemistry letters, 2013, 4(2): 249-253. 24900657
- Jones J E, Slack J L, Fang P, et al. Synthesis and screening of a haloacetamidine containing library to identify PAD4 selective inhibitors[J]. ACS chemical biology, 2011, 7(1): 160-165. 22004374