Protein-arginine deiminase type-4
| Basic Information | |
|---|---|
| Short Name | PAD4, HL-60 PAD |
| UNP ID | Q9UM07 |
| Organism | Homo sapiens |
| Cys Site | Cys645 |
| Family/Domain | Protein arginine deiminase family |
| Known Ligand | Ligand list |
| Function Type | Metabolic enzyme |
Summary
Protein Function
PAD4 is responsible for the conversion of arginine to citrulline residues. This protein may play a role in granulocyte and macrophage development leading to inflammation and immune response. PADI4 plays a role in the epigenetics, the deimination of arginines on histones 3 and 4 can act antagonistically to arginine methylation.
The protein may be found in oligomers and binds 5 calcium ions per subunit. It catalyses the reaction:
Protein L-arginine + H2O = protein L-citrulline + NH3 (From Wikipedia)
Cys Function & Property
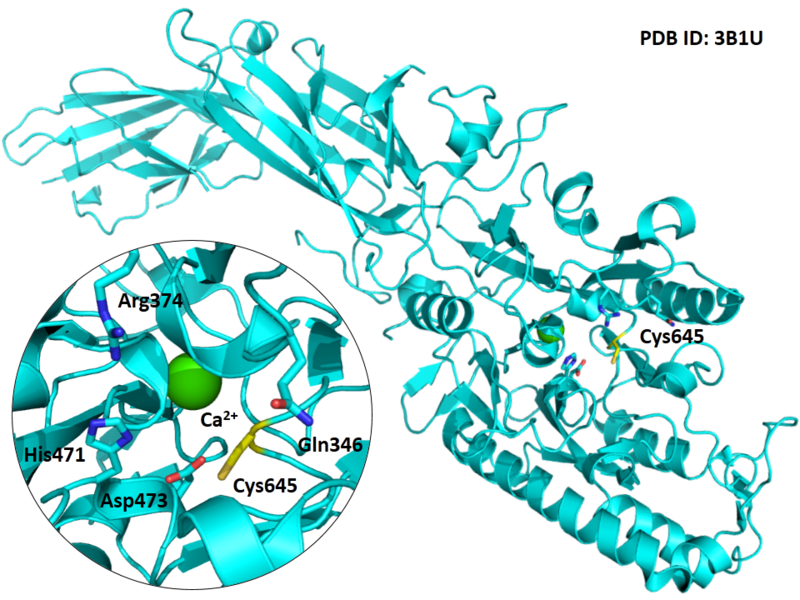
Cys645 is one of the active sites of PAD4.
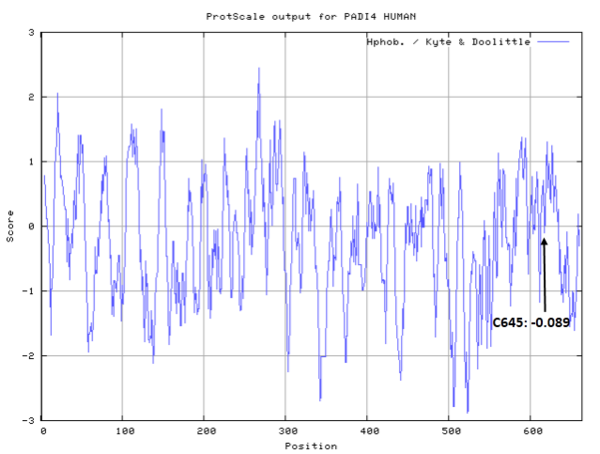
- Hydrophobic property:
- SASA:
- Cys645: 1.897 A^2
Protein Sequence
MAQGTLIRVT PEQPTHAVCV LGTLTQLDIC SSAPEDCTSF SINASPGVVV
DIAHGPPAKK KSTGSSTWPL DPGVEVTLTM KVASGSTGDQ KVQISYYGPK
TPPVKALLYL TGVEISLCAD ITRTGKVKPT RAVKDQRTWT WGPCGQGAIL
LVNCDRDNLE SSAMDCEDDE VLDSEDLQDM SLMTLSTKTP KDFFTNHTLV
LHVARSEMDK VRVFQATRGK LSSKCSVVLG PKWPSHYLMV PGGKHNMDFY
VEALAFPDTD FPGLITLTIS LLDTSNLELP EAVVFQDSVV FRVAPWIMTP
NTQPPQEVYA CSIFENEDFL KSVTTLAMKA KCKLTICPEE ENMDDQWMQD
EMEIGYIQAP HKTLPVVFDS PRNRGLKEFP IKRVMGPDFG YVTRGPQTGG
ISGLDSFGNL EVSPPVTVRG KEYPLGRILF GDSCYPSNDS RQMHQALQDF
LSAQQVQAPV KLYSDWLSVG HVDEFLSFVP APDRKGFRLL LASPRSCYKL
FQEQQNEGHG EALLFEGIKK KKQQKIKNIL SNKTLREHNS FVERCIDWNR
ELLKRELGLA ESDIIDIPQL FKLKEFSKAE AFFPNMVNML VLGKHLGIPK
PFGPVINGRC CLEEKVCSLL EPLGLQCTFI NDFFTYHIRH GEVHCGTNVR
RKPFSFKWWN MVP
Structural Information
- Known structure with covalent ligand:
- Protein structure:
Related Pathway
- Unknown
Experimental Evidence
- Crystallography, Cys-directed Mutation, MALDI-TOF MS
Reference
- Luo Y, Knuckley B, Lee Y H, et al. A fluoroacetamidine-based inactivator of protein arginine deiminase 4: design, synthesis, and in vitro and in vivo evaluation[J]. Journal of the American Chemical Society, 2006, 128(4): 1092-1093. 16433522
- Bello A M, Wasilewski E, Wei L, et al. Interrogation of the active sites of protein arginine deiminases (PAD1,-2, and-4) using designer probes[J]. ACS medicinal chemistry letters, 2013, 4(2): 249-253. 24900657
- Luo Y, Arita K, Bhatia M, et al. Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization[J]. Biochemistry, 2006, 45(39): 11727-11736. 17002273