Sodium- and chloride-dependent GABA transporter 1
| Basic Information | |
|---|---|
| Short Name |
SLC6A1, GABATR, GABT1, GAT1 |
| UNP ID | P30531 |
| Organism | Homo sapiens |
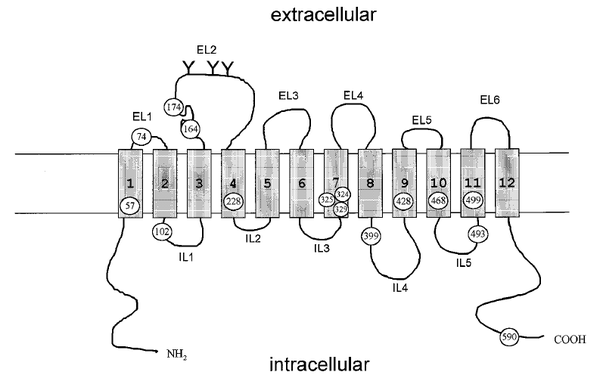
| Cys Site | Cys74 |
| Family/Domain | Sodium:neurotransmitter symporter (SNF) family, SLC6A1 subfamily |
| Known Ligand | Ligand list |
| Function Type | Transporter |
Summary
Protein Function
In the nervous system, removal of the inhibitory neurotransmitter c-aminobutyric acid (GABA) from the extracellular space of neurons and glia is accomplished by electrogenic Na+- and Cl--coupled GABA transporters (GATs). GAT1 was the first family member to be cloned by Guastella and Nelson in 1990. And it is the most abundant isoform within the nervous system and is expressed in both neurons and glia.
Terminates the action of GABA by its high affinity sodium-dependent reuptake into presynaptic terminals.
Cys Function & Property
Cys74, which located in the hydrophilic loop connecting transmembrane domains 1 and 2, is one of the extracellularly exposed, endogenous cysteine residue of GAT1, has been used to gain insight into the nature and functional consequences of sulfhydryl modification at this residue in many works, such as Bennett and Kanner 1997 (PMID: 8995422), Yu et al. 1998 (PMID:9599002), Golovanevsky and Kanner 1999 (PMID: 10438469), Li et al. 2000 (PMID: 10736315).
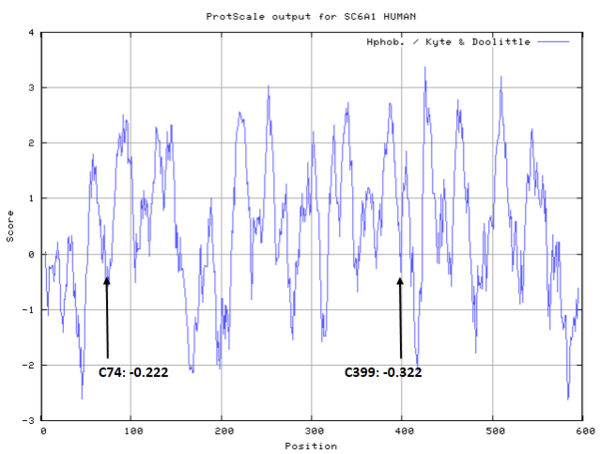
- Hydrophobic property:
- SASA:
- Unknown
Protein Sequence
MATNGSKVAD GQISTEVSEA PVANDKPKTL VVKVQKKAAD LPDRDTWKGR
FDFLMSCVGY AIGLGNVWRF PYLCGKNGGG AFLIPYFLTL IFAGVPLFLL
ECSLGQYTSI GGLGVWKLAP MFKGVGLAAA VLSFWLNIYY IVIISWAIYY
LYNSFTTTLP WKQCDNPWNT DRCFSNYSMV NTTNMTSAVV EFWERNMHQM
TDGLDKPGQI RWPLAITLAI AWILVYFCIW KGVGWTGKVV YFSATYPYIM
LIILFFRGVT LPGAKEGILF YITPNFRKLS DSEVWLDAAT QIFFSYGLGL
GSLIALGSYN SFHNNVYRDS IIVCCINSCT SMFAGFVIFS IVGFMAHVTK
RSIADVAASG PGLAFLAYPE AVTQLPISPL WAILFFSMLL MLGIDSQFCT
VEGFITALVD EYPRLLRNRR ELFIAAVCII SYLIGLSNIT QGGIYVFKLF
DYYSASGMSL LFLVFFECVS ISWFYGVNRF YDNIQEMVGS RPCIWWKLCW
SFFTPIIVAG VFIFSAVQMT PLTMGNYVFP KWGQGVGWLM ALSSMVLIPG
YMAYMFLTLK GSLKQRIQVM VQPSEDIVRP ENGPEQPQAG SSTSKEAYI
Structural Information
- Known structure with covalent ligand:
- Unknown
- Protein structure:
- Unknown
Related Pathway
Experimental Evidence
- Cys-directed mutation, Homologous Analysis Of Sequence, Molecular Docking
Reference
- Omoto J J, Maestas M J, Rahnama-Vaghef A, et al. Functional consequences of sulfhydryl modification of the γ-aminobutyric acid transporter 1 at a single solvent-exposed cysteine residue[J]. The Journal of membrane biology, 2012, 245(12): 841-857. 22918627