Tyrosine-protein phosphatase non-receptor type 1
| Basic Information | |
|---|---|
| Short Name | PTPN1, PTP1B |
| UNP ID | P18031 |
| Organism | Homo sapiens |
| Cys Site | Cys215, Cys121 |
| Family/Domain |
Protein-tyrosine phosphatase family, Non-receptor class 1 subfamily |
| Known Ligand | Ligand list |
| Function Type |
Phosphatase, Post-translational Modification |
Summary
Protein Function
PTP1B is a tyrosine-protein phosphatase which acts as a regulator of endoplasmic reticulum unfolded protein response. Mediates dephosphorylation of EIF2AK3/PERK; inactivating the protein kinase activity of EIF2AK3/PERK. May play an important role in CKII- and p60c-src-induced signal transduction cascades. May regulate the EFNA5-EPHA3 signaling pathway which modulates cell reorganization and cell-cell repulsion. May also regulate the hepatocyte growth factor receptor signaling pathway through dephosphorylation of MET.
Cys Function & Property
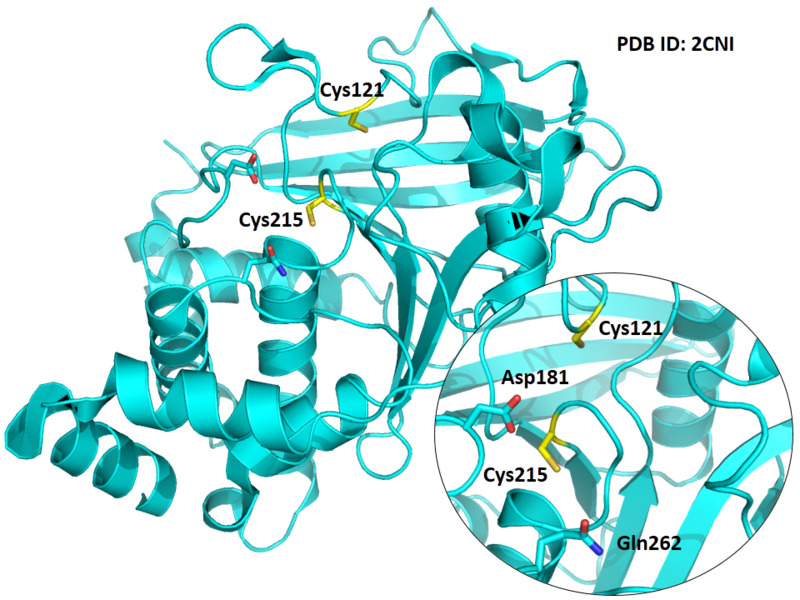
Cys215 is the active site of PTP1B.
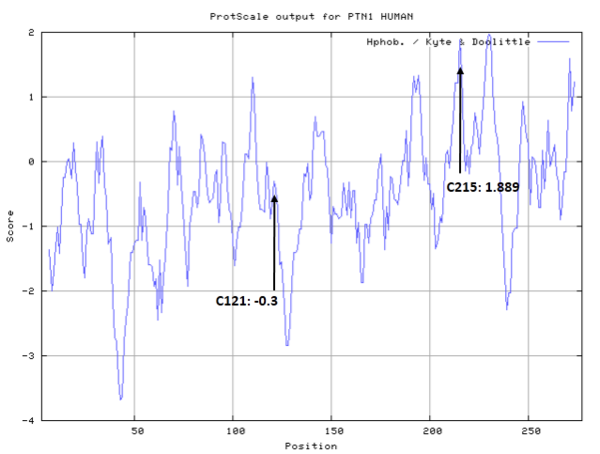
- Hydrophobic property:
- SASA:
- Cys121: 2.142 A^2
- Cys215: 2.483 A^2
Protein Sequence
MEMEKEFEQI DKSGSWAAIY QDIRHEASDF PCRVAKLPKN KNRNRYRDVS
PFDHSRIKLH QEDNDYINAS LIKMEEAQRS YILTQGPLPN TCGHFWEMVW
EQKSRGVVML NRVMEKGSLK CAQYWPQKEE KEMIFEDTNL KLTLISEDIK
SYYTVRQLEL ENLTTQETRE ILHFHYTTWP DFGVPESPAS FLNFLFKVRE
SGSLSPEHGP VVVHCSAGIG RSGTFCLADT CLLLMDKRKD PSSVDIKKVL
LEMRKFRMGL IQTADQLRFS YLAVIEGAKF IMGDSSVQDQ WKELSHEDLE
PPPEHIPPPP RPPKRILEPH NGKCREFFPN HQWVKEETQE DKDCPIKEEK
GSPLNAAPYG IESMSQDTEV RSRVVGGSLR GAQAASPAKG EPSLPEKDED
HALSYWKPFL VNMCVATVLT AGAYLCYRFL FNSNT
Structural Information
- Known structures with covalent ligands:
- Protein structure:
Related Pathway
Experimental Evidence
- Crystallography, LC-MS/MS, Tryptic Digest, Molecular Docking, MALDI-TOF MS, Cys-directed Mutation
Reference
- Krishnan N, Bencze G, et al. The anti‐inflammatory compound BAY‐11‐7082 is a potent inhibitor of protein tyrosine phosphatases[J]. The FEBS journal, 2013, 280(12): 2830-2841. 23578302
- Beei C, Iwamoto N, Inaba T, et al. Activation of EGFR/MEK/ERK/AP-1 signaling mediated by 1, 2-naphthoquinone, an atmospheric electrophile, in human pulmonary A549 cells[J]. The Journal of toxicological sciences, 2013, 38(5): 793-797. 24067727
- Abdo M, Liu S, Zhou B, et al. Seleninate in place of phosphate: irreversible inhibition of protein tyrosine phosphatases[J]. Journal of the American Chemical Society, 2008, 130(40): 13196-13197. 18781746
- Brandão T A S, Johnson S J, Hengge A C. The molecular details of WPD-loop movement differ in the protein-tyrosine phosphatases YopH and PTP1B[J]. Archives of biochemistry and biophysics, 2012, 525(1): 53-59. 22698963
- Brandão T A S, Hengge A C, Johnson S J. Insights into the Reaction of Protein-tyrosine Phosphatase 1B Crystal structures for transition state analogs of both catalytic steps[J]. Journal of Biological Chemistry, 2010, 285(21): 15874-15883. 20236928
- Sheriff S, Beno B R, Zhai W, et al. Small Molecule Receptor Protein Tyrosine Phosphatase γ (RPTPγ) Ligands That Inhibit Phosphatase Activity via Perturbation of the Tryptophan–Proline–Aspartate (WPD) Loop[J]. Journal of Medicinal Chemistry, 2011, 54(19): 6548-6562. 21882820